 [3]
[3] 

The Bone and Joint Decade, from 2000 to 2010, saw an increase in the awareness of and education for osteoporosis, as well as therapies used in the clinical management of osteoporosis and fragility fracture. Although cause and effect cannot be determined, over time epidemiologic studies reported a steady decline in fractures, particularly of the hip.1 However, with the emerging information on the serious, yet rare, side effects of long-term exposure to some of the osteoporosis medications (particularly Nitrogen-containing bisphosphonates), changes in care from the physician and patient level emerged in the field, which have led to what is now being called an osteoporosis treatment gap.2
In October 2010, as a result of reports associating long-term use of anti-osteoporosis medications (particularly the Nitrogen-containing bisphosphonates and also denosumab) with rare adverse events such as atypical femoral fractures (AFF) and osteonecrosis of the jaw (ONJ), the FDA issued revised guidance concerning long-term use of bisphosphonate treatments. An AFF starts as a weakening of the outer rim of the femur below the hip area as a tiny crack resembling a stress fracture. Unlike more common osteoporosis fractures, AFF develop over time from normal activities. If warning signs are not addressed, eventually the thigh bone may break completely. ONJ occurs when the jaw bone is exposed. Most cases of ONJ happen in long-term or very high dose bisphosphonate users after a dental extraction. The risk of ONJ in patients taking bisphosphonates for osteoporosis has a rate estimated between .001 and .01%.3 In October 2010, the FDA provided guidelines [1] on patient review for therapies used longer than three to five years, typically bisphosphonates, and suggested taking a break (or "holiday") from potent antiresorptive medications, including Nitrogen-containing bisphosphonates, in appropriate patients.
There is limited evidence to support both the practice of “drug holidays” and to determine the appropriate time to resume therapy, if one chooses to have a “drug holiday.” The use of DXA and bone turnover markers may assist in the clinical decision (e.g., a decline in bone mass density (BMD) greater than the smallest detectable difference in DXA or an increase in bone turnover markers beyond the normal premenopausal range); however, some patients never return to therapy. As previously reported in the Fracture Trends section, since 2012, the decline in hip fractures that was previously observed in older women has plateaued or even reversed in the US. This finding may be associated with an osteoporosis treatment gap that was highlighted earlier. The major key challenge for the future is for researchers to identify factors associated with the treatment gap, and work with patients on the best way to mediate them so physicians can continue to provide the best care for patients in reducing the risk for fractures.
Currently available data are not conducted at the person-level and do not link lifestyle factors or treatment with diagnosis; therefore, causal relationships cannot be established. However, there are many factors that may be associated with the current osteoporosis treatment gap, including (1) a decrease in DXA testing, (2) a decrease in the number of providers performing DXAs, and (3) the decreasing prevalence of women with osteoporosis diagnoses, which may contradict some of the epidemiologic data.
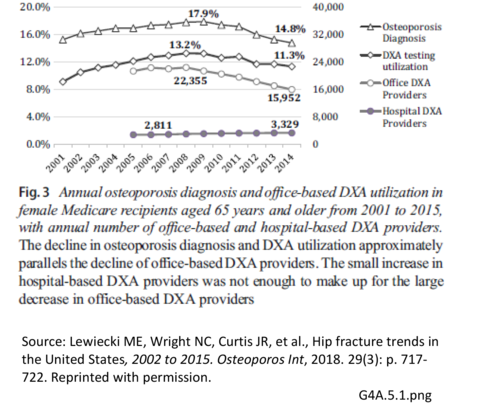
Depicted in the Figure below, the prevalence of DXA testing has decreased in the rate given to female Medicare recipients age 65 and older from a peak of 13.2% in 2008 to 11.3% in 2014. Likewise, the number of DXA providers, particularly office-based providers, has decreased from 22,355 in 2008 to 15,952 in 2014, a loss of 6,403 providers. This decrease was not offset by a slight rise of 518 hospital DXA providers between 2007 to 2014.4
Another potential factor is the decline in the use of medications. Drug holidays have become an option in treatment (see above), and along with the fear of side effects, there has been a decrease in the use of anti-osteoporosis medications. A study using commercial pharmacy data evaluated the number of dispensed prescriptions of both oral and parenteral bisphosphonates from 2002 to 2012. The total number of dispensed prescriptions reached a high of 31.0 million in 2008 and decreased to 14.7 million in 2012.5 Likewise, a study of Medicare patients with a hip fracture showed that the probability of osteoporosis medication use within 12 months of hip fracture discharge decreased from 40% in 2002 to 21% in 2011.6
To potentially understand the decline in medication use, researchers conducted ecologic studies describing medication use over time and evaluating the timing of the various FDA safety announcements. A study using commercial data evaluated the proportion of hip fracture patients using anti-osteoporosis medications.7 They stratified by bisphosphonates and all other osteoporosis medications. The investigators plotted the distribution of users over time and indicated when the FDA released announcements regarding safety around three adverse events: osteonecrosis of the jaw (May 2005), atrial fibrillation (Oct 2007), and atypical femur fractures (Mar 2010). The study, depicted in the graphic below, clearly showed that the FDA announcements may have had temporal associations with the use of bisphosphonates, but not apparently on the use of other osteoporosis medications. Specifically, the authors found a 4% decrease in the odds of bisphosphonate use in hip fracture patients every quarter after the release of the atypical femur fracture announcement, whereas there was no association in the other osteoporosis medications.8
Poor health education is another factor associated with the osteoporosis treatment gap. Although the Bone and Joint Decade increased the amount of osteoporosis education that was being circulated, it might not have reached all racial and ethnic groups. The prevalence of osteoporosis among Hispanic populations is equal to or higher than non-Hispanic whites, although the number of fractures is higher among non-Hispanic whites. Based on US Census data, the Hispanic population is the fastest growing ethnic group in the US overall, as well as among older adults,9,10 suggesting the need for culturally appropriate osteoporosis education information. Affiliates with the National Osteoporosis Foundation (NOF) have developed a Hispanic version of the NOF website to empower Hispanic women about osteoporosis, and “Fit to a T,” one of the patient education tools sponsored by the USBJI also sponsors Spanish language versions of several osteoporosis programs. This is one of the first steps in reducing disparities seen in DXA screening and medication use among Hispanic women.
Additional education is also needed for black women and the providers who serve them. Although black women have a generally lower prevalence of osteoporosis, those who have fractures have worse outcomes than their white counterparts. Several studies have shown that mortality after hip fractures is nearly 30% higher in black women than white women, even after adjusting for demographic, health, and surgical factors. Black women are less likely to be screened for osteoporosis,11, even high risk women who have sustained hip fractures,12 and less likely to receive medications.13,14,15 Although this disparity may have had a minimal impact on the current osteoporosis treatment gap, it still represents an area that needs improvement in the field.
Links:
[1] https://www.fda.gov/Drugs/DrugSafety/ucm229009.htm#safety
[2] https://bmus.latticegroup.com/file/bmuse4g4a51png
[3] https://bmus.latticegroup.com/docs/bmus_e4_g4a.5.1.png
[4] https://bmus.latticegroup.com/file/bmuse4g4a52png
[5] https://bmus.latticegroup.com/docs/bmus_e4_g4a.5.2.png
[6] https://bmus.latticegroup.com/file/bmuse4g4a53png
[7] https://bmus.latticegroup.com/docs/bmus_e4_g4a.5.3.png
[8] https://arapc.com/osteonecrosis-jaw-onj/
[9] https://www.census.gov/population/www/cen2000/briefs/phc-t9/tables/tab03.pdf
[10] https://factfinder.census.gov/faces/tableservices/jsf/pages/productview.xhtml?pid=ACS_15_1YR_S0103&prodType=table