

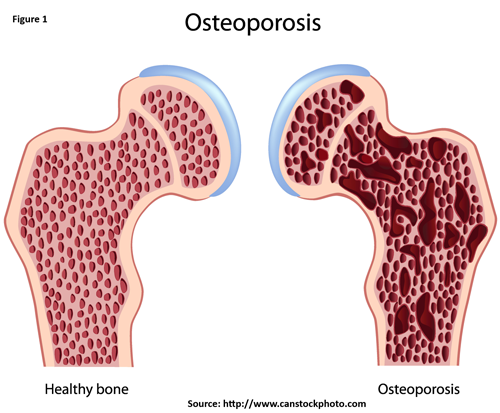
Osteoporosis is a skeletal disorder characterized by compromised bone disorder predisposing to an increased risk of fracture. It occurs when your body produces too little bone, there is a reduction in bone mass due to aging or other causes, or both (Figure 1).
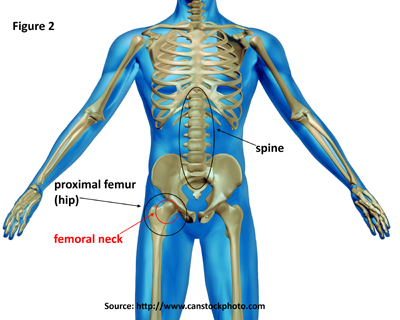
The primary diagnostic test for osteoporosis is bone mineral density (BMD). This test, which is generally taken at the proximal femur (hip) and spine (Figure 2), helps estimate the density of bones and the likelihood of breaking a bone. The hip, particularly the femoral neck, and spine are used because these are the most common sites for fractures. BMD is measured by a dual-energy X-ray absorptiometry (DXA). DXA testing provides an estimate of real BMD in g/cm2, and the estimate is converted into a T-score by comparing it to the BMD levels of a healthy young adult population. The BMD values of the 20- to 29-year-old females from the NHANES-III study (1988 to 1994) population are typically used as the reference population. Using thresholds developed by the World Health Organization (WHO), osteoporosis is defined as a T-score of -2.5 or less at either the lumbar spine or proximal femur (hip). T-scores between -2.5 and -1.0 identify individuals with low bone mass. T-scores greater than -1.0 represent normal bone mass.1
When BMD measurements are not available, diagnosis of osteoporosis is sometimes made based on fragility fractures, particularly with respect to the spine. The presence of vertebral fracture (VF) identifies a patient who has clinical osteoporosis; however, up to 75% of VFs are asymptomatic. Lifetime height loss of 1.5 inches or more also can be a sign of osteoporosis when BMD does not indicate osteoporosis or vertebral fractures are not present. One study found that 30% of men and women would have been misclassified (undiagnosed with osteoporosis) based on bone mineral density alone.2
In the United States, the national prevalence of osteoporosis is based on data from the National Health and Nutrition Examination Survey [1] (NHANES). NHANES is conducted by the National Center for Health Statistics, Centers for Disease Control and Prevention (CDC), to assess the health and nutrition status of a representative sample of the noninstitutionalized US population. Interviews of participants in the study are conducted in their homes. They receive standardized physical measurements, including BMD measurements via DXA, in mobile examination centers that are moved around the nation. The most recent national estimates of the prevalence of osteoporosis are based on femoral neck and spine BMD data from NHANES 2005–2010.
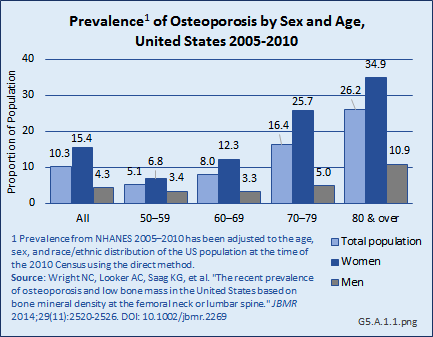
After adjusting for age, sex, and race/ethnicity, the prevalence of osteoporosis at either the femoral neck or lumbar spine is estimated to be 10.3% among adults age 50 years and older, representing more than 10 million people in the United States.1 (Reference Table 5.1 PDF [2] CSV [3])
Women have higher rates of osteoporosis at either of these two skeletal sites than men in the same age group across all ages: the prevalence of osteoporosis in adults age 50 years and older was 4.3% in men and 15.4% in women. Age is an even greater factor than sex in prevalence rates, particularly among women. Women ages 50 years to 59 years have a prevalence of 6.8%, while the prevalence increases to 34.9% for women age 80 years and older. Men show a similar, but less dramatic increase, with prevalence increasing from 3.4% among those ages 50 years to 59 years to 10.9% among men age 80 years and older. (Reference Table 5.1 PDF [2] CSV [3])
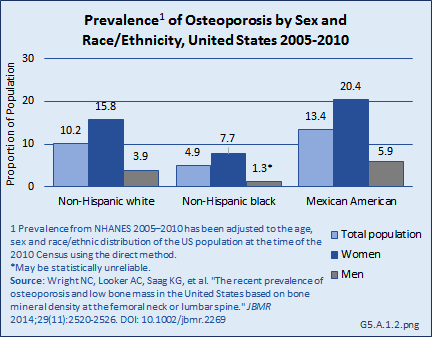
The prevalence of osteoporosis by race and ethnicity differs somewhat by BMD measurement site. Non-Hispanic Blacks have the lowest prevalence when based on BMD at either the hip or the spine, while Mexican Americans have the highest prevalence. However, when looking at the two skeletal sites separately, the group with the highest prevalence differs. Mexican Americans have the highest prevalence of osteoporosis if based on the lumbar spine BMD alone, but Non-Hispanic Whites have the highest prevalence if based on the femoral neck BMD alone.1 (Reference Table 5.1 PDF [2] CSV [3])
Using the current prevalence estimates of osteoporosis based on the NHANES BMD measurements at either the femoral neck or lumbar spine and future US Census projections, the number of people with osteoporosis will increase from an estimated 10.2 million in 2010 to 13.6 million people in 2030, with low bone mass, which may be a precursor to osteoporosis, increasing from 43.4 million people to 57.8 million over the same time frame.1
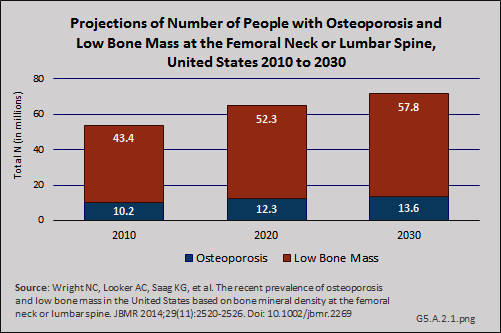
Although the prevalence of clinically diagnosed osteoporosis is projected to increase further, it is not clear whether the increase reflects an increase in diagnosis or an increase in the actual prevalence of the condition. Specifically, a comparison of femoral neck data from NHANES between 1988 and 1994 and 2005 and 2008 showed an increase in femoral neck BMD and a decline in prevalence of osteoporosis during this time. The observed differences in participant demographics and DXA methods between these NHANES surveys did not completely explain the observed increase in femoral neck BMD. Furthermore, data from NHANES 2005–2010 indicate that both mean femoral neck BMD and total lumbar spine BMD have remained stable during this five-year period among those age 50 years and older.
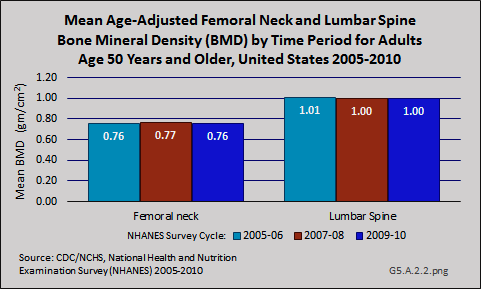
However, as noted in the introduction to osteoporosis, the diagnosis can also be made based on fragility fracture, which could also play a role in the discrepancy observed between secular trends in the prevalence of clinically diagnosed osteoporosis versus BMD-defined osteoporosis based on femoral neck data from NHANES.2
International Classification of Diseases (ICD) codes are often used in studies to define conditions in records of national health databases. However, health care providers may include codes that are not primary to the condition being treated, or may make errors in the coding process. Nevertheless, analysis of medical conditions based on ICD diagnosis or treatment codes is the most frequently used basis for health conditions research. Osteoporosis is identified with the ICD code of 733.
Data from the Agency for Healthcare Research and Quality Medical Expenditures Panel Survey [4] (MEPS) is used to estimate the economic burden [5] (cost) of musculoskeletal diseases throughout this site. The MEPS is the only US database to include actual cost paid by insurance companies and patients for the health care they receive.
Using the MEPS data, the prevalence of people with diagnosed osteoporosis has risen as the population ages. The number of persons in the population with an osteoporosis condition rose from 3.2 million to 6.5 million between 1996 and 1998 and 2009 and 2011, nearly doubling the number of people with osteoporosis. This number is lower than the 10 million estimated prevalence for people age 50 years and older reported based on BMD [6].
People between the ages of 45 years and 64 years experienced the steepest rise in osteoporosis diagnosis, increasing from 32% to 35% of all people with osteoporosis in the late 1990s to more than 40% in recent years. (Reference Table 10.1 PDF [7] CSV [8])
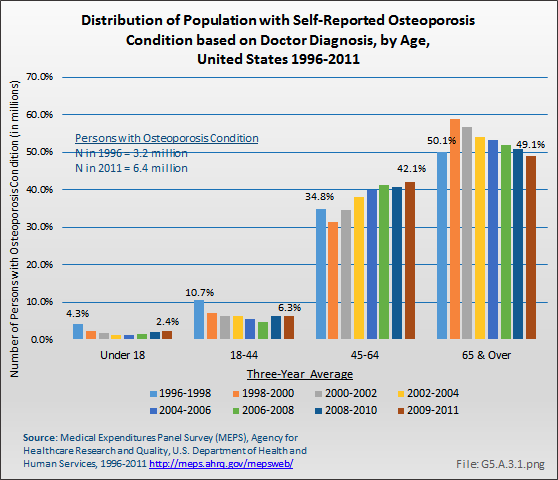
A recent report using Medicare data showed an overall increase of 18 percentage points in the number of Medicare beneficiaries with diagnosed osteoporosis, based on ICD-9-CM codes, between 2008 and 2010.1
Osteoporosis can be clinically diagnosed in individuals who have had a fragility fracture, irrespective of BMD,1 particularly if the fracture occurs at the hip or spine. Fragility fractures are used to measure the burden of osteoporosis, since osteoporosis in and of itself exhibits no clinical presentations such as pain or debility.
There is no one national database that captures the number of all hip and spinal fractures and estimates how many additional people have fractures without BMD-defined osteoporosis. The Centers for Disease Control and Prevention have estimated at least 250,000 people age 65 years and older are hospitalized for hip fractures each year, using the National Hospital Discharge Survey (NHDS). Nearly all (95%) hip fractures are caused by falling, usually falling sideways.1
Another comprehensive database for evaluation of the total number of hospitalized fractures is the Agency for Healthcare Research and Quality’s (AHRQ) Nationwide Inpatient Sample [10] (NIS), and is used throughout this site to estimate burden. The NIS includes more than 8 million inpatient hospitalizations each year from all payers in the United States, and is representative of 95% of all hospitalizations in the US. Among women 55 years and older, there were almost 1.7 million hospitalizations for fragility fractures in 2011. Hip fractures were the most common (23%) fractures requiring hospitalization, representing 325,200 hip fractures. Hospitalizations for clinically evident spine fractures (8.4%), representing 245,800 patients, was the second most common type of fragility fracture. For 92% of hospitalizations for hip fracture, this was the primary, or first, diagnosis; for spine fractures, 46% were the primary diagnosis. (Reference Table 5.2.1 PDF [11] CSV [12])
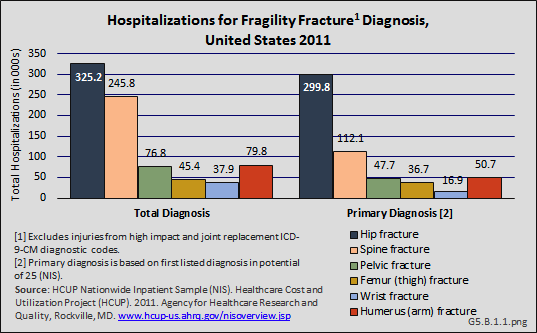
Relying on hospitalized fractures may underestimate the true prevalence of fragility fractures in the United States, since many fractures do not require hospital treatment and approximately two-thirds of all vertebral fractures are not clinically diagnosed.2 An analysis of the AHRQ NIS and National Emergency Department Sample (NEDS), and the CDC National Center for Health Statistics National Hospital Ambulatory Medical Care Survey (NHAMCS–Outpatient) and National Ambulatory Medical Care Survey (NAMCS–Physician Offices) for the years 2010 and 2011, showed 4.3 million fragility fracture visits, of which only 18% were hospital discharge visits. Wrist and arm fractures are the most likely fragility fractures to be treated outside a hospital. (Reference Table 5.2.1 PDF [11] CSV [12])
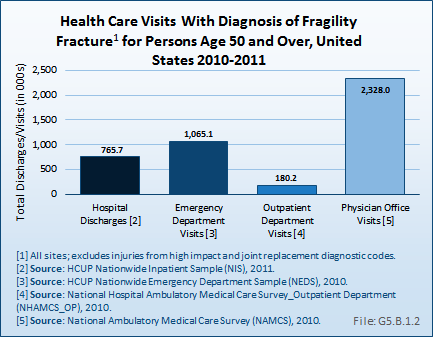
Women are more likely than men to have an osteoporosis diagnosis and to have a fragility fracture. Hospital discharges in 2011 with a fragility fracture diagnosis for women were more than twice the number of discharges in men with the same diagnosis (404,300 discharges for women; 159,500 discharges for men). This difference is evidenced for fragility fractures at all sites, and is also representative of emergency department (ED) visits with a fragility fracture diagnosis.
Age also is a major factor among people diagnosed with a fragility fracture, with people aged 80 years and older representing twice the rate of those between the ages of 70 and 79 years.
Overall, hospital discharges for fragility fractures accounted for 2.6% of hospital discharges for any diagnosis in 2011, and 2.0% of all ED visits. Among women, fragility fractures accounted for 3.5% of all hospital discharges and 2.6% of all ED visits. Among people aged 80 years and older, fragility fractures accounted for 6.4% of all hospital discharges and 4.9% of all ED visits. (Reference Table 5.2.2 PDF [14] CSV [15])
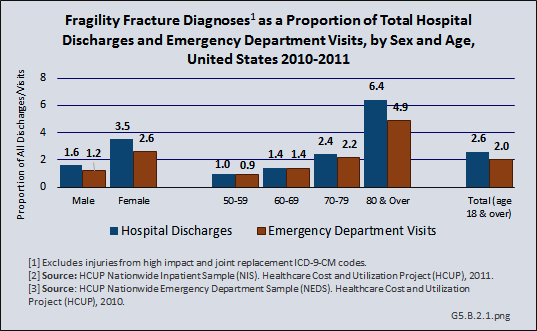
A more detailed discussion of sex and age differences in osteoporosis and fragility fractures can be found in the Special Populations section of this site in Sex and Gender [16] and Aging [17].
Although the number of hospital discharges for hip fractures has increased in recent years due to an aging population, several studies indicate a decreasing rate of hip fractures among white women and men, both in the United States and worldwide, by as much as an annualized 1.5% per year since the early 1990s.1,2,3,4,5 One recent study reports a reduction in the 10-year hip fracture probability for the white population, based on an updated study of hospital discharges using the National Inpatient Sample (NIS).6
The most recent national examination of hip fracture trends shown a decline in the age-adjusted incidence rate of fracture of 24.5% in women and 19.2% in men from 1995 to 2005.7 Racial and ethnic differences in the declines in hip fractures incidence have been evaluated. For example, a Medicare population study of hip fractures between 2000 and 2009 reported statistically significant declines in White men and women; however, due to smaller numbers, a decline among Black and Hispanic women is not statistically supported.8 Another study of hip fractures among Hispanics reported an increase in hip fractures in this population.9 Given these declines, the US version of the 10-year fracture probability calculator, FRAX, has been undated.
While declines in hip fracture incidence is being observed, fractures remain a concern because there is some indication the reduction in hip fractures is being offset with fractures in other sites, particularly vertebral fractures, due in large part to extended longevity.4
Osteoporosis-related fractures are associated with a substantial increase in the utilization of health services for both acute and long-term care. Fracture risk increases with age,1,2 as does the burden of disease in terms of morbidity, mortality, and costs.3 Here we focus on the effects of osteoporosis and related fractures on health care utilization.
While the incidence of hip fracture appears to have declined overall since the mid 1990s, as discussed in a previous section [18], hospital discharges from the MEPS database related to osteoporosis were 44% greater in 2009 to 2011 than they were in the years from 1996 to 1998. Recent years, however, have shown a decline after peaking in 2001 to 2003. (Reference Table 10.2 PDF [19] CSV [20]) This increase in hospitalizations likely reflects the increase in older population cohorts as the Boomer generation enters the prime years for fracture incidence.
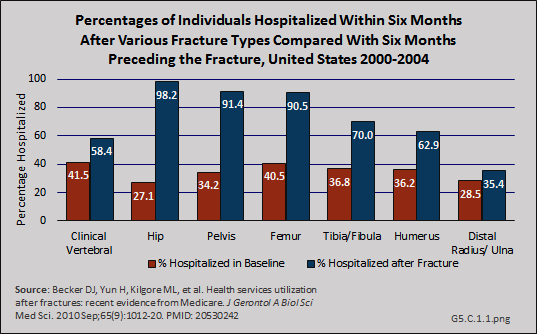
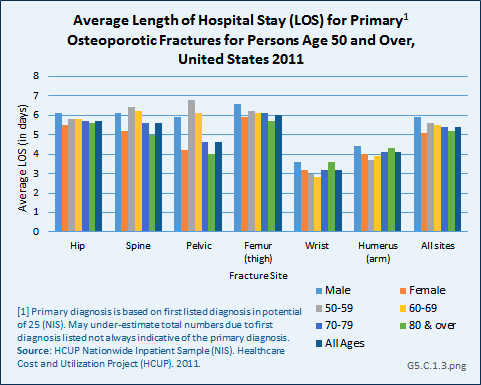
In a study among Medicare beneficiaries, the 65 years and older population, experiencing hip fractures in the years 2000 to 2004, 98.2% were hospitalized in the six months following the fracture compared to 27.1% hospitalized at least once in the prior six months for other reasons.1 Thus, hip fractures were associated with a 71% increase in the probability of being hospitalized. The average length of stay for hip and upper leg (femur) fractures was more than 11 days. (Reference Tables 5.3 PDF [21] CSV [22] and 5.4 PDF [23] CSV [24])
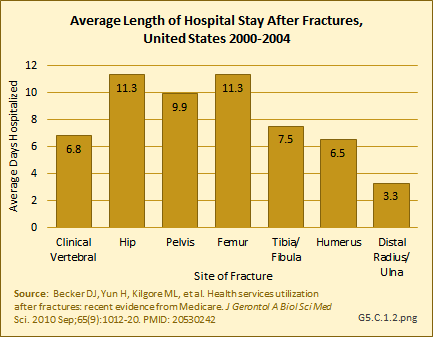
Using recent 2011 data from the NIS hospital discharge database, an average hospital stay of 5.5 days was reported for people age 50 years and older with a primary (first) diagnosis of fragility fracture. While it might be assumed the inclusion of younger-age patients could account for this difference from the earlier study, age was not a factor in length of stay. Sex was a more significant factor, with males staying nearly a day longer than females. With the exception of wrist and arm fractures, site of the fracture was also not a factor. (Reference Table 5.5 PDF [25] CSV [26])
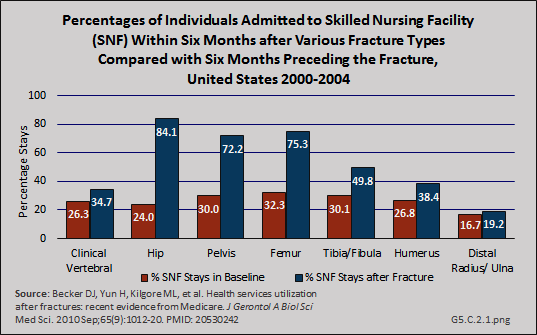
Patterns similar to hospitalization for the Medicare population were reported for use of skilled nursing facility and home health services following hospitalization before and after fractures, although the rates for these services were somewhat lower. Hip fractures were associated with an 84% increase in probability of a stay in a skilled nursing facility.1 (Reference Tables 5.3 PDF [21] CSV [22] and 5.4 PDF [23] CSV [24])
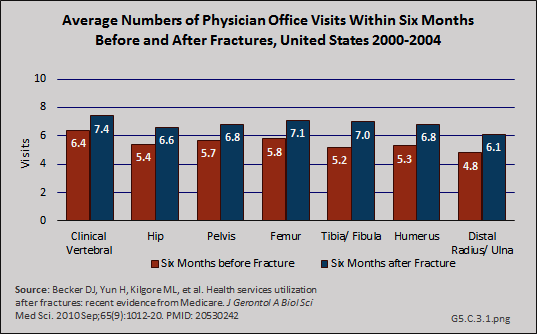
In a recent study, the effect of fracture on physician office visits using a baseline of visits six months before fracture and comparing it to six months after fracture, was less than for hospitalization, but still substantial.1 Fractures of the tibia/fibula had the greatest increase in physician office visits (35% increase in probability of a visit), but this could be because of higher rates of transfer to nursing home care for hip, pelvis, and femur fractures shown in studies discussed previously. (Reference Table 5.6 PDF [27] CSV [28])
Following fractures, utilization of physical and occupational therapy services increased by similar proportions.1
Although the mean number of ambulatory physician visits for osteoporosis care from the MEPS study, the data upon which estimated cost burden from osteoporosis [5] is based, declined between 1996 and 1998 and between 2009 and 2011, the total number of visits over this timeframe increased by 67% because of the larger number of people with osteoporosis. Because the MEPS data is based on only an osteoporosis diagnosis rather than a fracture diagnosis, the actual numbers may be larger than reported.
Again, using the MEPS, the mean number of home health visits for osteoporosis between 1996 and 1998 was 7.7; from 2009 to 2011 it was 7.4. However, the total number of visits rose from 24.3 million to 47.5 million due to the rise in reported prevalence of osteoporosis. Ambulatory nonphysician health care visits nearly tripled, increasing from 10.7 million to 28.9 million, due to an increase in both mean number of visits and the share of persons with reported osteoporosis who made at least one such visit. (Reference Table 10.2 PDF [19] CSV [20])
Serious adverse outcomes following hip fractures include mortality, debility requiring institutional care, and destitution (poverty) sufficient to qualify for Medicaid enrollment. In a recent study of Medicare beneficiaries, the population age 65 years and older, individuals experiencing hip fracture had more than double the risk of death, an almost four-fold increased risk of becoming a nursing home resident, and more than double the risk of enrolling in Medicaid within one year of the fracture.1 (Reference Table 5.7 PDF [29] CSV [30])
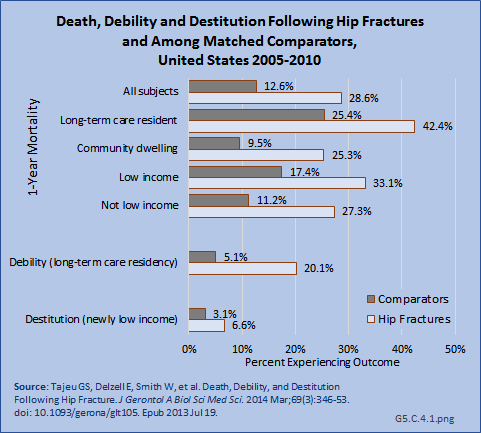
Two recent studies of the Medicare population support a significant decline in mortality from hip fractures in recent years, with reported mortality rates of 22%2 and 30%3 in the first year following fracture. However, hip fracture remains a major mortality risk 2- to 8-fold higher than non-hip-fracture matched controls.3
Health care treatments and visits contribute to the economic burden of osteoporosis, with increasing visits to physicians, to ambulatory nonphysician health care sites, and for home health services.
Overall, ambulatory care visits accounted for the largest share of per-person direct cost for people with an osteoporosis condition. At an average cost of $3,758 per person between 2009 and 2011, an increase of 42% from 1996 to 1998, ambulatory care accounted for 34% of per-person direct cost between 2009 and 2011. Both the share of per-person cost for inpatient care and the mean cost dropped between 1996 and 1998 to 2009 and 2011, with the share dropping from 36% to 24% and the mean cost from $3,009 to $2,681. However, the average per-person cost for prescriptions rose from $1,477 to $2,989, in 2011 dollars, an increase of 102%. (Reference Table 10.4 PDF [31] CSV [32])
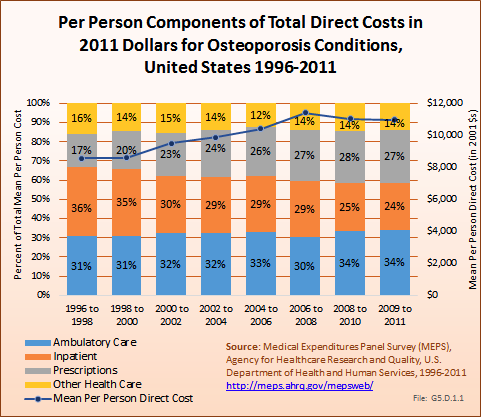
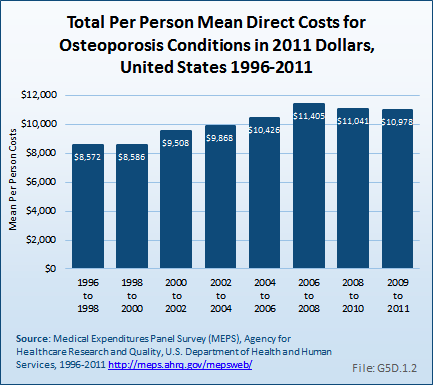
Total direct per-person health care costs in 2009 to 2011 for people with an osteoporosis condition were $10,978, an increase of 28% since 1996 to 1998. Incremental direct per-person costs, those costs most likely attributable to an osteoporosis condition, were not calculated for osteoporosis because of the small sample size. (Reference Table 10.6 PDF [33] CSV [34])
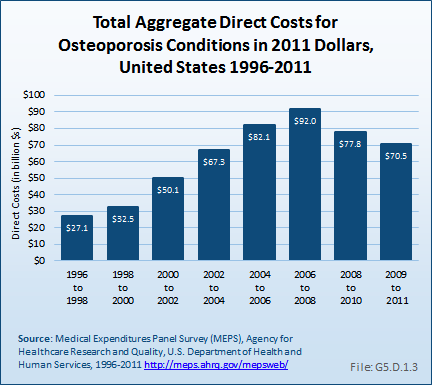
Total aggregate direct costs for persons with an osteoporosis condition were $70.5 billion from 2009 to 2011, a rise of 160% from the $27.1 billion from 1996 to 1998, in 2011 dollars.
Indirect costs associated with lost wages for people ages 18 years to 64 years are not calculated for those with an osteoporosis condition. Osteoporosis is rarely cited as a reason for lost workdays or bed days, in part because of the older age of the most commonly affected adults. However, approximately 50% of people with hip fractures do not regain their prior activity level, leading to societal costs for added care. Similarly, vertebral compression fractures due to osteoporosis contribute to spinal deformity, reduced mobility, and the need for assistance with activities of daily living, which increases the indirect societal costs.
There are many challenges in osteoporosis healthcare and delivery that need to be addressed in the future, including but not limited to screening, pharmacotherapy use, pharmacotherapy adherence, and addressing osteoporosis care in patients with multiple comorbidities.
Screening and treating at-risk patients can prevent fractures and reduce the morbidity associated with osteoporosis. Over the last few years, rates of osteoporosis screening via DXA testing have steadily declined in the US. The initial decline in 2006 was associated with reductions in the DXA reimbursement rates by Medicare. Fewer DXA scans were performed in 2007 and 2008 than the number projected using Commercial and Medicare Supplemental Insurance data from 2000 to 2006,1 and similar declines were observed in Medicare Fee-for-Service population.2 More recently, DXA examination rates were studied in women aged 50 years to 64 years, and the authors found reductions in the number of DXA scans performed between 2006 and 2012 (-35 DXAs per 1,000 patient years) in this younger population, with the most significant reduction (-33%) in the 50- to 54-year age group.3
The reduction in overall DXA screening is also affecting the high-risk fracture patient population. The use of DXA postfracture is low. In one Midwestern county hospital, only 10% of hip fracture patients had a DXA ordered upon hospital discharge.4 Only 10% of Medicare beneficiaries who sustained a major osteoporotic fracture received postfracture DXA testing,5. Four well-established Midwestern health care systems reported less than one-quarter of patients who had a hip fracture had bone density testing before or after their fracture.6 This data was corroborated by national Healthcare Effectiveness Data and Information Set (HEDIS) measures, demonstrating that less than one-third of people postfracture have received testing or treatment.7
Currently, the pharmacotherapies approved for the treatment and prevention of osteoporosis fall under two categories: antiresorptive and anabolic agents. The antiresorptive agents inhibit the action of bone cells that degrade bone, whereas the anabolic agents promote bone formation. Teriparitide is the only approved anabolic agent. There are a number of FDA- approved classes of antiresporptive agents including bisphosphonates, selective-estrogen receptor modulators (SERMS), receptor activator of nuclear factor kappa beta (RANK) ligand inhibitors, and estrogen. The bisphosphonates are the most commonly used agents, which include oral (alendronate, ibandronate, and risedronate) and parenteral (ibandronate and zoledronic acid) preparations.
All of these agents have been shown to reduce the risk of vertebral fracture. Some of these have been shown also to reduce the risk of nonvertebral fractures, in some cases including hip fracture. Large randomized, placebo-controlled studies suggest that there is a 30% to 70% reduction in the risk of vertebral fractures and a 16% to 25% reduction in the risk of nonvertebral fractures with pharmacologic treatment.1,2 A number of general recommendations can be made regarding pharmacologic therapy for osteoporosis:
Bisphosphonates are usually considered first-line treatment for osteoporosis, particularly because of their positive effects on vertebral, nonvertebral, and hip fractures.
Second-line options include denosumab, raloxifene, strontium ranelate (in countries where available), and teriparatide.
Teriparatide use is often confined to high-risk patients, those with fractures, and in those with glucocorticoid- induced osteoporosis. Risk of osteosarcoma limits administration to two years.
Largely in light of the Women’s Health Initiative (WHI) study results and the availability of many other efficacious compounds, estrogen is no longer considered first-line therapy.
Combination use of antiresorptive drugs is generally not recommended because there is no current evidence of additional antifracture benefits, and there may be an increased risk of side effects and the over-suppression of bone turnover.
Patients deemed appropriate candidates for a pharmacologic therapy include:
Men and women who have experienced a hip or spine fracture
Those with osteoporotic range BMD determined by DXA
Persons with low bone mass (osteopenia) meeting a fracture risk (FRAX) threshold of more than 3% for hip and more than 20% for major osteoporotic fracture, based on the National Osteoporosis Foundation (NOF) recommendations3
Selected individuals without low bone mass but high fracture risk due to risk states such as use of glucocorticoids
Fracture risk thresholds may be different outside the United States.
Although a number of agents are available, the use of osteoporosis pharmacotherapy is also declining. Using commercial dispensing data, the use of oral bisphosphonates, the most commonly prescribed anti-osteoporosis pharmacotherapy, decreased by more than 50% from a peak of 31 million prescriptions dispensed in 2007 to only 14.7 million in 2012.4 Although less drastic, the sales of parenteral bisphosphonates for osteoporosis have also declined by 22% since its peak of 561,600 units in 2010 to 436,900 in 2012.4 It is speculated that declining treatment rates may be associated with a decrease in osteoporosis screening, lower physician initiation of therapy, and/or patient- or provider-initiated “drug holidays,” which are becoming more common because of perceived safety concerns of long-term bisphosphonate use.
The declining use of pharmacotherapy is also prevalent among those newly diagnosed with osteoporosis. In a recent study among women with incident osteoporosis, more than 64% of women had no indications of osteoporosis pharmacotherapy use one year after diagnosis.5 Pharmacotherapy use in those who have sustained a fracture is low. Recent evaluations have found that only 12% of Medicare beneficiaries had evidence of osteoporosis pharmacotherapy use six months after sustaining a fracture.6 In a managed care population, only 18% of fracture patients had indications for osteoporosis pharmacotherapy within 90 days of their fracture, and only 23% appeared to use osteoporosis pharmacotherapy within a year postfracture.7 Studies in hip fracture patients found that 19% to 24% received therapy within one year postfracture,8,9 and that pharmacotherapy use posthip fracture has declined from 40% in 2002 to 21% in 2011.9
Low adherence to osteoporosis pharmacotherapies has been documented since bisphosphonates first became available in 1995. Less than 50% of patients starting an oral bisphosphonate are still receiving this drug one year later.1 The early formulations had strict dosing instructions and significant gastrointestinal side effects. The availability of parenteral preparations (intravenous [IV] zoledronic acid and ibandronate) and later formulations improved adherence rates, but even with these agents, adherence rates are not at 100%. Only 32% of IV zoledronic acid users received their second annual dose, and nearly 20% of IV ibandronate users received only one of the four annual doses.2 Mechanisms to improve adherence to medications include engaging pharmacists in patient education activities to encourage adherence.3,4 In Kaiser Permanente Colorado, an interactive voice response (IVR) reminder system followed by a personalized letter for patients prescribed osteoporosis medications led to increased medication purchase in the short term but did not change the medication adherence in the long term.5 Additional efforts targeting patients, providers, and the health system are needed to improve adherence to osteoporosis medications.
Multiple comorbidities create further challenges in the care of osteoporosis patients. Certain comorbidities and the medications used to treat them could also negatively impact bone health, for example, rheumatoid arthritis and the corticosteroids used in symptom relief. A recent study evaluated the comorbidities of women with and without osteoporosis in the Geisinger Health System.1 Of the comorbidities commonly found among women with osteoporosis compared to women without osteoporosis, two have known independent and/or drug-related adverse effects on bone. Gastroesophageal reflux disease (GERD) was found in 55.6 per 1,000 person-years in women with osteoporosis versus 40.3 per 1,000 person-years in those without, while depression was found in 46.8 versus 36.9 per 1,000 person-years in the respective groups.1 Diagnosed depression,2,3 depressive symptoms,4,5,6,7,8 and medications used to treat depression9,10,11,12,13 have been associated with osteoporosis and fractures. Proton Pump Inhibitors (PPIs) are a common GERD treatment. The use of PPIs, particularly at high doses, has been associated with higher overall fracture risk14,15,16,17,18 and hip fracture risk.19,20,21 Like the prevalence of osteoporosis, the number of comorbidities increases with aging. Comorbidities compete with health care and medication priorities and may contribute to the low adherence levels to osteoporosis drugs.
While the burden of osteoporosis is growing significantly, the declining pattern of osteoporosis testing and treatment has created considerable unmet need and clear opportunities for new treatments and programs. Even after a fracture, many healthcare providers do not discuss osteoporosis with their patients. Indeed, fractures beget fractures: Fractures at nearly any site are significantly associated with subsequent fractures,1,2 emphasizing the need for new drugs, treatment care approaches, and research funding to test the effectiveness of new therapies and treatment approaches.
There are three new drugs being tested as potential osteoporosis treatments.
Abaloparatide
Abaloparatide is an analog of parathyroid hormone-related protein (PTHrP) being developed as a potential anabolic agent for osteoporosis treatment. In a phase 2 study, abaloparatide increased BMD at the lumbar spine, femoral neck, and total hip in a dose-dependent fashion. The abaloparatide-induced BMD increases either trended to or were significantly greater than those seen with teriparatide. Phase 3 studies will evaluate fracture outcome and additional safety with this therapy.
Odanacatib
Odanacatib is a selective inhibitor of cathepsin K, a collagenase produced by osteoclasts and responsible for the degradation of bone mineral and matrix. Women receiving odanacatib for five years gained BMD at the spine and hip, and showed bone biomarker changes somewhat different from those seen with bisphosphonates and typical antiresorptive therapies.1,2 Also in contrast with bisphosphonates, after odanacatib discontinuation there was rapid reversal of treatment effects. Although the safety profile has been mild, cathepsin inhibitors have been associated with a scleroderma-like skin lesion. Other potential safety signals are being further investigated.
Anti-sclerostin antibodies
Sclerostin is produced by osteocytes and inhibits anabolic signaling pathway important for bone formation. Rare natural deficiencies in sclerostin result in sclerosing bone diseases, sclerosteosis, and van Buchem disease, which are radiographically characterized by thickened bones, including all long bones. Monoclonal antibodies to sclerostin are being investigated for both osteoporosis treatment and for fracture healing. Preliminary studies suggest a significant increase in BMD with the anti-sclerostin romosuzumab.3 Phase 3 clinical trials evaluating fracture risk protection are underway.
Coordination of acute postfracture care with subsequent secondary osteoporosis prevention and treatment is a tenet of good osteoporosis management. Although most people who experience a fracture receive excellent and appropriate acute care management in a hospital or emergency department, most are not subsequently referred to or do not pursue postfracture osteoporosis care with a bone health specialist.
The fracture liaison service (FLS) is a model of care developed in Europe and successfully implemented in a number of US managed health care systems that seeks to facilitate postfracture care coordination. FLS operates under the supervision of bone health specialists and collaborates with primary care, orthopedic, and emergency department health care providers. Care typically is coordinated postfracture through a nurse or other allied health professional. Patients with recent fractures are tracked via a registry, and timelines are established for postfracture assessments and follow-ups. FLS programs recognize that patients who have fractured are at highest risk of future fractures. FLS programs in certain settings have reduced the number of fractures and have achieved cost savings by identifying and appropriately treating postfracture patients.
The efficiency of the FLS approach varies depending on its intensity and potentially the location of its implementation.1,2
The National Institutes of Health (NIH) supports both basic and clinical investigation in osteoporosis. Within the NIH, the National Institute of Arthritis, Musculoskeletal, and Skin Diseases (NIAMS); the National Institute on Aging (NIA); the National Institute of Dental and Craniofacial Research (NIDCR); National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK); and The National Institute of Child Health and Human Development (NICHD) all support osteoporosis-related research. NIAMS [39], the top institute for osteoporosis research, reported $72 million in osteoporosis research funding in fiscal year 2014, with an NIH-wide total of $141 million for osteoporosis-related projects.1 Additional research support is provided by other federal agencies including the Agency for Health Care Research and Quality [40] (AHRQ), the Centers for Disease Control and Prevention [41] (CDC), and the Veterans Administration Research [42] program. The new Patient-Centered Outcomes Research Institute [43] (PCORI) has also supported osteoporosis research. The top private and agency funds for osteoporosis include the American Society of Bone and Mineral Research [44]and the American College of Rheumatology [45]. Although numerous agencies fund osteoporosis research, the dollars available are limited in comparison to other conditions prevalent in older Americans. Additional research funding would assist in identifying treatments, management strategies, and factors that can minimize the burden associated with osteoporosis.
OSTEOPOROSIS
Osteoporosis unspecified: 733.00
(ICD-10-CM: M81.0)
Senile osteoporosis: 733.01
(ICD-10-CM: M81.0)
Idiopathic osteoporosis: 733.02
(ICD-10-CM: M81.8)
Disuse osteoporosis: 733.03
(ICD-10-CM: M81.8)
Other osteoporosis: 733.09
(ICD-10-CM: M81.8)
FRAGILITY FRACTURES
Hip fracture: 820.0, 820.2, 733.14
(ICD-10-CM: S72.019A, S72.023A, S72.033A, S72.043A, S72.099A, S72.109A, S72.143A, S72.23XA, M84.459A)
Spine fracture: 805.0, 805.2, 805.4, 805.8, 806.0, 806.2, 806.4, 806.8, 733.13
(ICD-10-CM: S12.9XXA, S12.000A, S12.001A, S12.100A, S12.101A, S12.200A, S12.201A, S12.300A, S12.301A, S12.400A, S12.401A, S12.500A, S12.501A, S12.600A, S12.601A, S22.009A, S32.009A, S32.10XA, S32.2XXA, S14.101A, S14.102A, S14.103A, S14.104A, S14.111A, S14.112A,S14.113A, S14.114A, S14.121A, S14.122A, S14.123A, S14.124A, S14.131A, S14.132A, S14.133A, S14.134A, S14.151A, S14.152A, S14.153A, S14.154A, S14.105A,S14.106A, S14.107A, S14.115A, S14.116A, S14.117A, S14.125A, S14.126A, S14.127A, S14.135A, S14.136A, S14.137A, S14.155A, S14.156A, S14.157A, S24.101AS24.102A, S24.111A, S24.112A, S24.131A, S24.132A, S24.151A, S24.152A, S24.103A, S24.104A, S24.113A, S24.114A, S24.133A, S24.134A, S24.153A, S24.154A,S34.109A, S34.119A, S34.129A, S32.009A, S34.101A, S34.111A, S34.121A, S32.019A, S34.102A, S34.112A, S34.122A, S32.029A, S34.103A, S34.113A, S34.123A,S32.039A, S34.104A, S34.114A, S34.124A, S32.049A, S34.105A, S34.115A, S34.125A, S32.059A, S14.109A, S24.109A, S34.109A, S34.139A, M48.50XA, M80.08XA,M84.48XA, M84.68XA)
Pelvic fracture: 808.0, 808.2, 808.4, 808.8
(ICD-10-CM: S32.409A, S32.501A, S32.501A, S32.509A, S32.309A, S32.609A, S32.810A, S32.811A, S32.82XA, S32.89XA, S32.9XXA)
Femur (thigh) fracture: 821.0, 821.2, 733.15
(ICD-10-CM: S72.90XA, S72.309A, S72.409A, S72.413A, S72.416A, S72.443A, S72.446A, S72.453A, S72.456A, S72.499A, M84.453A)
Wrist fracture: 813.4, 733.12
ICD-10-CM: S52.90XA, S52.539A, S52.549A, S52.509A, S52.609A, S52.119A, S52.529A, S52.019A, S52.629A, S52.011A, S52.012A, S52.621A, A52.622A, M84.439A)
Humerus (arm) fracture: 812.0, 812.2, 812.4, 733.1
(ICD-10-CM: S42.209A, S42.213A, S42.216A, S42/293A, S42.295A, S42.253A, S42.256A, S42/293A, S42.296A, S42.309A, S42.399A, S42.409A, S42.413A, S42.416A, S42.433A, S42.436A, S42.453A, S42.456A, S42.443A, S42.446A, S42.463A, S42.466A, S42.473A, S42.476A, S42.493A, S42.496A, M84.40XA)
Links:
[1] http://www.cdc.gov/nchs/nhanes.htm
[2] https://bmus.latticegroup.com/docs/T5.1.pdf
[3] https://bmus.latticegroup.com/docs/T5.1.csv
[4] http://meps.ahrq.gov/mepsweb/
[5] http://www.boneandjointburden.org/2014-report/x0/economic-cost
[6] http://www.boneandjointburden.org/2014-report/va2/projected-prevalence
[7] https://bmus.latticegroup.com/docs/T10001.1.pdf
[8] https://bmus.latticegroup.com/docs/T10001.1.csv
[9] http://dx.doi.org/10.5888/pcd11.130118
[10] https://www.hcup-us.ahrq.gov/nisoverview.jsp
[11] https://bmus.latticegroup.com/docs/T5.2.1.pdf
[12] https://bmus.latticegroup.com/docs/T5.2.1.csv
[13] http://www.cdc.gov/homeandrecreationalsafety/falls/adulthipfx.html
[14] https://bmus.latticegroup.com/docs/T5.2.2.pdf
[15] https://bmus.latticegroup.com/docs/T5.2.2.csv
[16] http://www.boneandjointburden.org/2014-report/ixae0/osteoporosis
[17] http://www.boneandjointburden.org/2014-report/ixbe0/osteoporosis-aging-population
[18] http://www.boneandjointburden.org/2014-report/vb3/hip-fracture-trends
[19] https://bmus.latticegroup.com/docs/T10003.2.pdf
[20] https://bmus.latticegroup.com/docs/T10003.2.csv
[21] https://bmus.latticegroup.com/docs/T5.3.pdf
[22] https://bmus.latticegroup.com/docs/T5.3.csv
[23] https://bmus.latticegroup.com/docs/T5.4.pdf
[24] https://bmus.latticegroup.com/docs/T5.4.csv
[25] https://bmus.latticegroup.com/docs/T5.5.pdf
[26] https://bmus.latticegroup.com/docs/T5.5.csv
[27] https://bmus.latticegroup.com/docs/T5.6.pdf
[28] https://bmus.latticegroup.com/docs/T5.6.csv
[29] https://bmus.latticegroup.com/docs/T5.7.pdf
[30] https://bmus.latticegroup.com/docs/T5.7.csv
[31] https://bmus.latticegroup.com/docs/T10005.4.pdf
[32] https://bmus.latticegroup.com/docs/T10005.4.csv
[33] https://bmus.latticegroup.com/docs/T10007.6.pdf
[34] https://bmus.latticegroup.com/docs/T10007.6.csv
[35] http://www.ncqa.org/ReportCards/HealthPlans/StateofHealthCareQuality.aspx
[36] http://dx.doi.org/10.1016/j.clinthera.2015.03.023
[37] http://dx.doi.org/10.1136/bmj.e372
[38] http://dx.doi.org/10.1016/j.maturitas.2014.05.019
[39] http://projectreporter.nih.gov/reporter.cfm
[40] http://www.ahrq.gov/
[41] http://www.cdc.gov/grants/
[42] http://www.research.va.gov/funding/
[43] http://www.pcori.org/
[44] http://www.asbmr.org/Grants/Default.aspx
[45] http://www.rheumatology.org/
[46] http://report.nih.gov/categorical_spending.aspx