

Although somewhat uncommon by comparison to such diseases as lung, breast, and kidney cancers, musculoskeletal neoplasms cause significant morbidity and mortality in patients. This is especially true in young patients who are more likely to develop such cancers as osteosarcoma, Ewing sarcoma and rhabdomyosarcoma. Musculoskeletal neoplasms and sarcomas, in particular, usually require concerted treatment efforts by coordinated medical teams. These teams are typically led by a subspecialty of physicians known as orthopedic oncologists. Because of the relative infrequency of musculoskeletal sarcomas, few institutions gather sufficient numbers to provide thorough epidemiologic and descriptive data. Therefore, tumor registry data are necessary to gather enough cases to generate meaningful data.
The following discussion is based on concerted analysis that incorporates the two largest tumor registries in the United States, The National Cancer Data Base (NCDB) of the American College of Surgeons [1](ACS) and the National Cancer Institute’s Surveillance, Epidemiology and End Results (SEER) [2] program. Actual incidence, death rates, and survival statistics are difficult to determine. The two databases derive slightly different numbers, and the numbers change annually with newly reported data. Thus, a direct comparison of NCDB and SEER is not possible. Sources for data cited in tables and graphs are shown. Sources available at the time of analysis may no longer be available.
The NCDB is a joint project of the Commission on Cancer of the American College of Surgeons and the American Cancer Society. The data used in this study and this report are derived from a de-identified NCDB file comprising over 1500 Commission-accredited cancer programs. The American College of Surgeons and the Commission on Cancer have not verified and are not responsible for the analytic or statistical methodology employed, or the conclusions drawn from these data by the investigator and authors of this chapter.
We also derived some tumor incidence estimates by analysis and extrapolation from one of the author’s case series data derived from his practice experience. Dr. William Ward was the only orthopedic oncologist at Wake Forest University Baptist Medical Center in Winston Salem, NC, during the period between 1991 and 2005. Virtually all cases of osteosarcoma in North Carolina were treated by one of the very few orthopedic oncologists in North Carolina during that time. Dr. Ward’s personal surgical database contains detailed incidence data regarding many musculoskeletal neoplasms. Comparing the incidence of osteosarcomas in the United States with that treated by Dr. Ward, we were able to extrapolate, using similar proportional estimates, to the national incidence of tumors for which there were no national registry data. Typically, aggressive benign bone and soft tissue tumors are most likely to be treated by orthopedic oncologists rather than by non-oncological–trained surgeons. However, because there is no way to estimate the numbers of patients treated by orthopedic surgeons not specifically trained in oncology, the derived national data estimates will be conservative because of the methodology used. All estimates in this chapter derived from the above methodology will be clearly identified as an extrapolation of incidence estimation.
All tissues are made up of individual cells. Tumors, also known as neoplasms, are the result of excessive abnormal growths of cells that multiply and divide without control. In malignant tumors, the tumor cells multiply, divide, and spread. If unchecked, malignant tumors can cause death as they spread, or metastasize, to vital areas of the body. Benign tumors, on the other hand, do not spread or metastasize to other body locations. However, they can cause significant local injury or disease at the site of the primary tumor. Tumors, therefore, are growths of new tissue that are uncontrolled and progressive.
Muscle, bone, nerves, blood vessels, fat, and fibrous tissues are all connective tissues.
Primary bone and soft tissue tumors originate in bone or connective tissue rather than spread to bone or connective tissue from another site. Secondary tumors are those that began elsewhere and spread (metastasize) to the bone or connective tissues. Primary tumors can be benign, which means they do not spread through the body to other sites, or malignant (cancerous), meaning they can and do spread to other places in the body. Malignant tumors of the bone and connective tissue are also known as sarcomas, unlike cancers in other organs, which are generally referred to as carcinomas.
Most musculoskeletal cancers, or sarcomas, are named by the Latin root word for the type of malignant tissue they produce. Thus, osteosarcomas are malignant bone (osteo) cells, chondrosarcomas manufacture malignant cartilage (chondro) cells, liposarcomas make malignant fatty tissue (lipo) cells, rhabdomyosarcomas create malignant muscle tissue (rhabdomyo) cells, fibrosarcomas produce malignant connective tissue (fibro), and so on.
Secondary bone tumors are those that spread to the bone from malignancies in other organs such as lung, breast, and prostate cancers. These are known as metastatic cancers, and are far more numerous than primary bone cancers. Although metastatic cancers to bone cause extensive morbidity from pain and fractures caused by bone weakening, such cancers are not the primary focus of this chapter. However, a following section on secondary bone and joint cancers details some of the effects of this condition and its associated morbidity.
Benign tumors are neoplasms that do not tend to spread, or metastasize, to other sites and therefore are not cancerous. They rarely threaten the life of the patient although they can cause significant injury at the site of the tumor.
The incidence of cancer is defined as the number of new cancers of bone and connective tissue in a specific population during a year. The incidence rate is expressed as the number of cancers per 100,000 population at risk. In general, it does not include recurrences. Because of the low number of new cases, the incidence rate in this report is expressed as the number per one million population at risk.
Prevalence is defined as the number of people alive on a certain date in a population who have the disease and have previously had a diagnosis of the disease. It includes new (incidence) cases and pre-existing cases, and is a function of past incidence and survival.
A cancer mortality rate is the number of deaths, with cancer as the underlying cause of death, occurring in a specific population during a year. It is calculated the same as the incidence rate.
Cancer survival statistics are typically expressed as the proportion of patients alive at some point subsequent to the diagnosis of their cancer.1 Relative survival is a net survival measure representing cancer survival in the absence of other causes of death. Relative survival is defined as the ratio of the proportion of observed survivors in a cohort of cancer patients to the proportion of expected survivors in a comparable set of cancer-free individuals.2 Observed survival is the actual percentage of patients still alive at some specified time after diagnosis of cancer. It considers deaths from all causes, cancer or otherwise. Lifetime risk is the probability of developing or dying from cancer in the course of one's lifetime.3
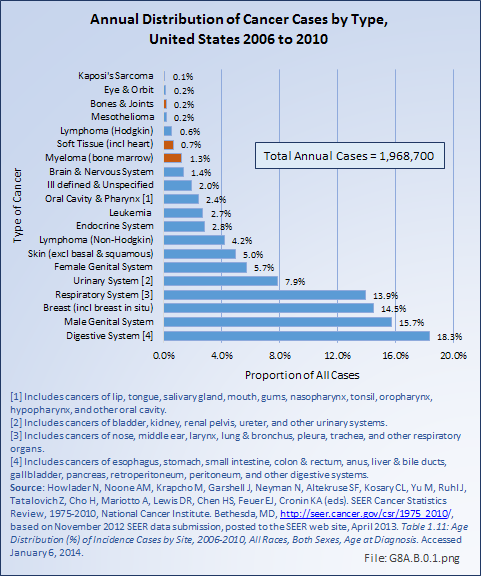
Bone and connective tissue neoplasms, which include bone and joint sarcoma, myeloma, and soft tissue sarcomas, are uncommon when compared with other cancers and with other musculoskeletal conditions, accounting for about 2.2% of annual cancer cases between 2006 and 2010 (approximately 43,000 cases). This is slightly higher than the rate of 1.9% reported previously for the years 2002 to 2006. The annual average number of new bone and joint cancer cases between 2006 and 2010 was 3,888, while during this same time an average of 1,360 deaths from bone and joint cancer occurred each year. (Reference Table 8A.1.1 PDF [6] CSV [7], Table 8A.3.1 PDF [8] CSV, [9] and Table 8A.3.3 PDF [10] CSV [11])
Data from the Surveillance Epidemiology and End Results (SEER) [12] program of the National Cancer Institute, used to present the burden of bone and connective tissue neoplasms, is the most comprehensive source of information on cancer incidence, prevalence, mortality, survival, and lifetime risks. Data is currently available from 1974 to 2006. The SEER program is one of the most comprehensive sources of neoplasm data; it is based on data representing approximately 10% of the US population.
In addition, data from the American College of Surgeons’ Commission on Cancer National Cancer Data Base (NCDB) [1] was used in the analysis for staging and growth rates for common cancers. The NCDB is maintained by the American College of Surgeons, containing much of the same standardized data as that collected by the SEER database. Data are collected from all institutions wishing to be accredited by the American College of Surgeons Commission on Cancer. Each accredited institution is required to report all patients with cancer treated at their institution, including annual follow-up data. Site visits and interaction between American College of Surgeons cancer database personnel and the local reporting institutions verifies a minimum of 90% case capture and reporting for each institution. Multiple internal checks verify the data accuracy. It is estimated that the approximately 1,500 reporting institutions each year treat approximately 71% of all patients with malignancies in the United States. The primary author was granted research access to the database under the Participant User File (PUF) research program. However, accessible data was only for cases in patients 18 years old and older, showing the age-related aspects of the NCDB dataset.
The three most common primary cancers of bones and joints are osteosarcoma, Ewing sarcoma, and chondrosarcoma. Of the three, chondrosarcoma has the best prognosis. The ages at which these cancers most often occur vary. Osteosarcoma, a malignant bone tissue tumor commonly found near the growing end of the long bones, is the most common, and occurs most frequently in teens and young adults. Ewing sarcoma, a tumor often located in the shaft of long bones and in the pelvic bones, occurs most frequently in children and youth. Chondrosarcoma, a sarcoma of malignant cartilage cells, often occurs as the result of malignant degeneration of pre-existing cartilage cells within bone, including chondromas (a benign tumor), and is primarily found among older adults. However, the vast majority of chondromas never undergo malignant change; therefore, the routine resection of benign chondromas is unwarranted.
Of these three, Ewing sarcoma is generally considered to have the worst prognosis, followed by osteosarcoma. However, this perception is largely due to the greater tendency for osteosarcomas to present as high-grade tumors and for chondrosarcomas to present as low-grade tumors. When analyzed by stage, a recent survivorship analysis revealed similar survivorship rates for low-grade chondrosarcoma compared to low-grade osteosarcoma, and similar survivorship rates for high-grade chondrosarcoma when compared to high grade osteosarcoma. By definition, all cases of Ewing sarcoma are high-grade, the most aggressive category of cancer, with full potential to metastasize and bring about death. Approximately 6% to 20% of osteosarcomas are of lower grade; chondrosarcoma has a higher proportion of low-grade cases than these other two bone and joint cancers.
The fourth common “primary” cancer of the bone is myeloma, a malignant primary tumor of the bone marrow formed from a type of bone marrow cells called plasma cells (the cells that manufacture antibodies). This cancer and usually involves multiple bones simultaneously. The isolated single-bone version of myeloma is called plasmacytoma, but virtually all cases of isolated plasmacytoma evolve into full-fledged multiple myeloma within 5 to 10 years after diagnosis of the plasmacytoma. Like leukemia and lymphoma, myeloma is more properly considered a primary cancer of the hematopoietic bone marrow. Unlike leukemia, however, myeloma typically causes extensive changes or damage to the bone structure itself, causing fractures, pain, and hypercalcemia. Because of the associated bone destruction, myeloma is generally included in analysis of bone cancers; however, leukemia and lymphomas generally are not considered primary bone cancers, presumably because of the lower likelihood of structural bone destruction and associated complications. Non-Hodgkin’s lymphomas, however, warrant some consideration due to the frequency of bone destruction and pathological fractures requiring operative intervention.
SEER estimated that in 2013, 3,010 people were newly diagnosed with cancer of the bones and joints. The number of new cases of bone and joint cancers is 0.9 per 100,000 people per year. In addition, 1,440 people will die annually from cancer of the bone and joints.1 According to SEER statistics, the rates for new bone and joint cancer cases have been rising, on average, 0.4% each year over the last ten years. However, death rates have fallen on average 0.3% per year, with survival rates rising slightly. Incidence estimates derived from NCDB are slightly higher without an appreciable change in survival. There has been a gradual increase in the number of cases reported to the NCDB in the past 13 years. While the reason for this difference is unknown, it could reflect reporting changes rather than incidence changes.
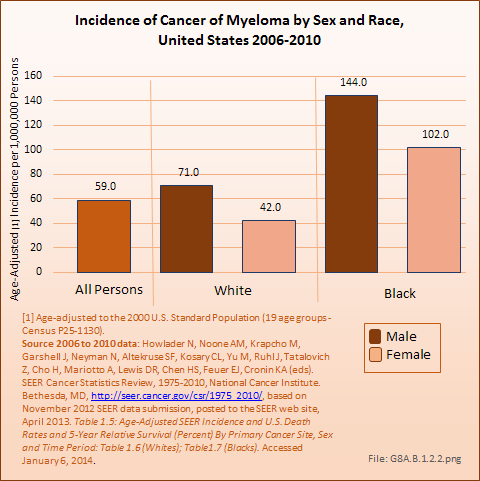
Myeloma occurs five to six times as frequently as the other bone cancers. It will be diagnosed in 22,350 persons per year, an incidence rate of 5.9 per 100,000 persons per year. An expected 10,710 persons died of myeloma in 2013.2
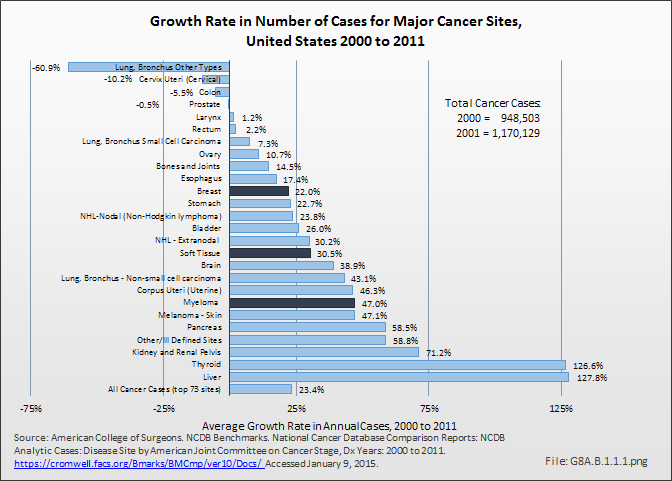
Although annual cases included in the American College of Surgeons National Cancer Data Base (NCDB) show a slower rate of increase, proportionately, for bone and joint cancers than for cancer cases overall, the incidence of bone and joint cancers is increasing. Between 2000 and 2011, the annualized number of primary cases recorded increased by nearly 15%. Myeloma cases, however, increased at twice the rate of cancer cases overall, 47% to 23%, respectively. (Reference Table 8A.5.2 PDF [13] CSV [14])
Gender and Race
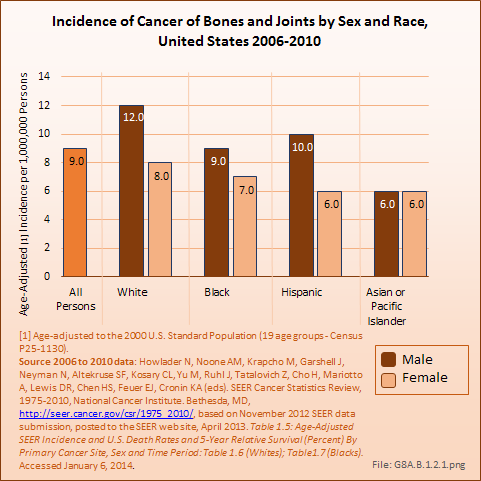
Bone cancers and soft tissue sarcomas are found more frequently in males than females, and more frequently among Whites than those of any other race. However, rates have varied slightly for both genders and by race for the past decade. The average annual incidence of bone cancers between 2006 and 2010 was nine in one million, a rate that has remained constant for the last decade. The rate among White males was 12 in one million, while, among White females it was eight in one million. The lowest rate of six in one million was found for both males and females of the Asian or Pacific Islander race. The incidence of cancer of the bones and joints in the United States is comparable to several site-specific oral cancers (ie, lip, salivary gland, floor of the mouth), cancers of the bile duct, cancers of the eye, and Kaposi’s sarcoma, which affects the skin and mucous membranes and is often associated with immunodeficient individuals with AIDS. (Reference Table 8A.1.1 PDF [6] CSV [7])
As with bone and joint cancers, males have a higher incidence of myeloma than do females, with an average of 71 cases in one million males to 42 cases in one million females. Blacks have a much higher incidence rate of myeloma than Whites. The incidence of myeloma in the United States is comparable to the incidence of esophageal, liver, cervical, ovarian, brain, and lymphocytic leukemia cancers. (Reference Table 8A.1.2 PDF [17] CSV [18])
Age
The median age for cancers of the bones and joints has risen slightly, to age 42 years, in recent years. However, it remains the leading cause of cancer in young persons under the age of 20 years. More than one in four diagnoses of bone and joints cancer is in children and youth under the age of 20 years, with more than one-half (52%) of cases diagnosed in person younger than 45 years. Males are typically diagnosed with bone cancers, and die from bone cancer, at an age several years younger than females. (Reference Table 8A.2.1 PDF [19] CSV [20] and Table 8A.3.2 PDF [21] CSV [22])
Myeloma, on the other hand, is primarily a cancer found among elderly persons, with a median age of 69 at the time of diagnosis. Eight-five percent of new myeloma cases are diagnosed in persons age 55 years and older. Again, males are typically diagnosed with myeloma at ages several years younger than females. (Reference Table 8A.2.1 PDF [19] CSV [20], Table 8A.2.2 PDF [23] CSV [24], and Table 8A.3.2 PDF [21] CSV [22])
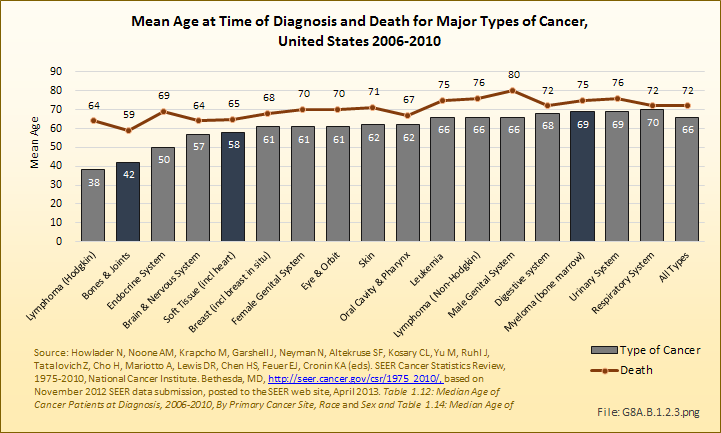
Death from bone and joint cancer, at a median age of 59 years, occurs at a younger age than any other type of cancer. (Reference Table 8A.2.2 PDF [23] CSV [24])
Annual population-based mortality rates due to cancers of bones and joints are low, averaging four deaths per one million people since the early 1990s.1 While the mortality rate from bone and joint cancer dropped by approximately 50% from that of the late 1970s, no significant improvement in this rate has been observed over the past 20 years.2 Males have a higher mortality rate than females for all races. (Reference Table 8A.4.3 PDF [25] CSV [26])
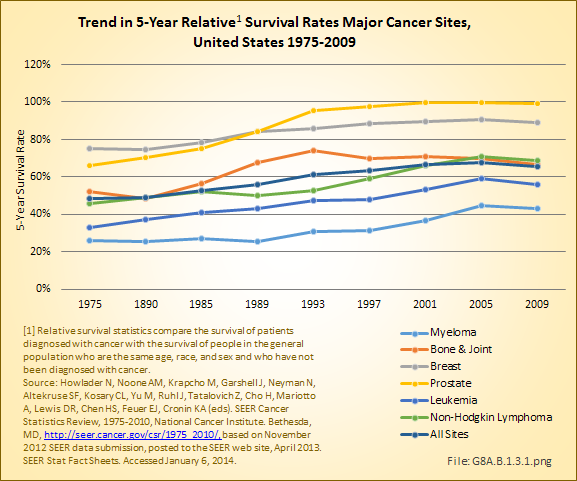
The overall 5-year survival rate in 2006–2010 for bone and joint cancers was 66%, placing it roughly in the middle of all cancers for 5-year survival and comparable to a number of more common cancers such as non-Hodgkin lymphoma, urinary, cervical/ovarian, and soft tissue cancers. This is an increase of 14% since 1975, when the 5-year survival rate was 52%. The median number of years of survival after diagnosis is 17, with males averaging 16 years and females 18 years.
The overall 5-year survival rate for the primary types of bone and joint cancers is 54% for osteosarcoma, 75% for chondrosarcoma, and 51% for Ewing sarcoma. The osteosarcoma survival rate varies with age: The 5-year survival was 70% for children and youth under the age of 20 years,3 60% for people under 30 years of age, 50% for those aged 30 years to 49 years, and 30% for those 50 years old and older.2 If Ewing sarcoma is found before it metastasizes, the 5-year survival rate for children and youth is about 70%. However, if already metastasized when found, the 5-year survival rate drops to 15% to 30%.4 (Reference Table 8A.4.1 PDF [27] CSV [28], Table 8A.4.2 PDF [29] CSV, [30] and Table 8A.4.3 PDF [25] CSV [26])
The annual population-based mortality rate of myeloma was an average of 34 persons per one million population between 2006 and 2010.5 The mortality rate from myeloma has remained relatively constant since the mid-1970s. The 5-year survival rate for myeloma, 43%, is one of the lowest for all cancers; however, due to being primarily a cancer of older persons, this age-relatedness may well affect survival regardless of the presence or absence of myeloma. The median survival after diagnosis of myeloma is only 6 years. (Reference Table 8A.4.1 PDF [27] CSV [28], Table 8A.4.2 PDF [29] CSV, [30] and Table 8A.4.3 PDF [25] CSV [26])
Within the NCDB, no change in the overall survival rates for patients diagnosed and treated in the years 1985 to 1988 compared to patients between 1994 and 1998 was found. There have been no substantial changes in therapies utilized for osteosarcoma since 1998 and the overall 1998–2010 NCDB data reveals no significant improvement, with an approximate 50% five-year overall survival. However, the survival rate varies greatly with the histologic subtype of sarcoma. For instance, the 5-year relative survival rate is 53% for classic high-grade osteosarcoma, 87% for parosteal osteosarcoma and 18% for osteosarcoma associated with Paget's disease of the bone. (Reference Table 8A.8.1 PDF [31] CSV [32])
The economic burden of bone cancers can be great. The more advanced the disease, the worse the prognosis and, accordingly, the more expensive the treatments. It is likely that early detection, and, certainly, prevention if possible, could drastically reduce costs. A number of expensive treatments are required to address these tumors. In the 2007 report by Damron, Ward, and Stewart,1 it was noted that the most frequent initial treatments varied widely based on the type of sarcoma. Although not reported, these treatments vary widely based on the stage of the disease as well.
Collectively, they reported that surgery alone was the most common initial treatment for chondrosarcomas (69%), whereas for Ewing sarcoma, treatments were divided between surgery and chemotherapy (24% of cases), radiation and chemotherapy (23%), and chemotherapy alone in 18%. With osteosarcoma, when initial treatment was known, the largest group received surgery and chemotherapy (46%). Surgery was reported as part of the initial treatment in 71% of osteosarcoma patients, 83% of chondrosarcoma patients, and 47% of Ewing sarcoma patients. The most frequent operations performed were limb-sparing radical resections and excisions. When the type of surgery was defined and known, limb-preservation surgery was performed in 69% of osteosarcomas, 79% of chondrosarcomas, and 81% of Ewing sarcomas.1
Radiation therapy was employed in 10% of osteosarcomas, 14% of chondrosarcomas, and 46% of Ewing sarcoma cases. In Dr. Ward’s personal series of more than 100 osteosarcomas, amputation has been required in only 17% of patient, but almost all patients have had surgical resection, limb reconstruction, and chemotherapy.1
Multiple therapies may be needed later in the course of the patient’s disease, especially in the more advanced cases. In later stages of the disease for those not cured with surgery alone, significant costs will accumulate as the patients develop pulmonary disease and, ultimately, die. Hormone therapy, immunotherapy, and bone marrow transplant/endocrine treatments each accounted for 1% or less of initial treatments. However, in severely affected individuals in whom standard treatments fail, these alternative treatments may be tried more frequently. Currently, the authors are not aware of any data source that reports the rate of utilization of such late treatments.
All of these treatments are costly to administer. Per-patient cost will vary widely depending on the treatments utilized, and the number and intensity of treatments. Over all, treatment for bone and joint cancers can easily exceed $100,000 for a single patient. This is particularly true if that patient receives surgery, chemotherapy, and radiation therapy. If one includes the cost of bone-replacing endoprostheses or the costs of artificial limbs used in those cases that required amputations, the cost will be much higher. In addition to the direct medical cost, there are extensive indirect and social costs from lost work time and disability. For some patients, healthcare costs associated with their bone and joint cancers will be ongoing.
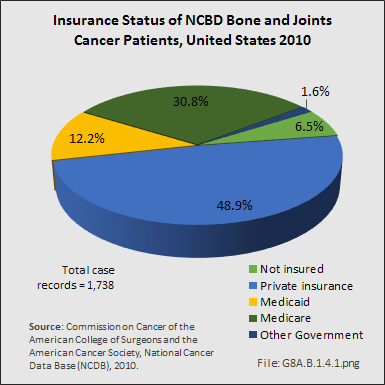
The burden of paying the cost of treatment for bone and joint cancers is shifting. In the study cited above, from 1998 to 2010, managed care provided insurance coverage for the largest portion of patients (37%), following by Medicare with supplement (16%). Medicare and Medicaid were roughly equal at 8% and 8%, respectively. However, analysis of the NCDB insurance data available for 1,738 (95.7%) of 1,816 bone and joint cancer cases reported for 2010 show Medicare and Medicaid covering a much larger share.
Almost all cancers have preferential sites to which they spread or metastasize, resulting in secondary cancers. Secondary bone cancer is much more common than primary bone cancers, and result in great morbidity and pain. The three most common sites of cancer metastasis are lung, liver, and bone. The skeleton is the most common organ affected by metastatic cancer, and the site of disease that produces the greatest morbidity. The most commonly encountered cancers that readily and frequently spread to bone are cancers of the breast, lung, kidney, prostate, gastrointestinal tract, and thyroid gland. The incidence of bone metastases in lung cancer patients is approximately 30% to 40%, and the median survival time (MST) of patients with such metastases is 6 to 7 months.1 At postmortem examination, 70% of patients dying of breast and prostate cancer have evidence of metastatic bone disease. Cancers of the thyroid, kidney, and bronchus also commonly give rise to bone metastases, with an incidence at postmortem examination of 30% to 40%.2 Brain and ovarian cancers rarely spread to bone. Many other cancers have intermediate rates of spread to bones.
A tumor formed by metastatic cancer cells is called a metastatic tumor or a metastasis. The cancer cells in their new metastatic site closely resemble the original or primary cancer from which the cancer initially arose. For example, breast cancer that spreads to the bone and forms a metastatic tumor is still considered metastatic breast cancer, not true bone cancer. It will still look like breast tissue and breast cancer when it is inspected or viewed under a microscope. Many lay people will now refer to it as bone cancer, but to the physician, bone cancer implies a cancer that started or originated in the bone, such as osteosarcoma, Ewing sarcoma, or myeloma, as discussed above.
Metastatic bone disease complications are termed skeletal-related events (SREs). SREs include pain, pathologic fracture, vertebral deformity and collapse, spinal cord compression, and hypercalcemia (overabundance of calcium in the blood) of malignancy. These complications result in impaired mobility and reduced quality of life (QOL) and have a significant negative impact on survival.2 In addition, metastatic disease may remain confined to the skeleton, with the decline in quality of life and eventual death almost entirely due to skeletal complications and their treatment.
The prognosis of metastatic bone disease is dependent on the primary site, with breast and prostate cancers associated with a survival measured in years compared with lung cancer, where the average survival is only a matter of months. Survival rates for secondary bone cancer depend on patient factors such as age, overall health, treatment, and response to treatment. However, due to the advanced stage of cancer that has spread, survival rates are much lower than for primary cancer without such spread.
The fundamental treatment for bone metastasis from advanced cancer is disease control by systemic chemotherapy and radiation of the bone lesions. Prevention and treatment of bone metastases is highly dependent on an effective treatment being employed against the primary cancer. As a direct treatment for bone metastases themselves, radiation therapy, surgery, and bisphosphonates are the mainstays of treatment. Intravenous bisphosphonates, such as zoledronic acid have been shown to prevent or reduce pathologic fractures and may reduce these costs.3 With FDA approval of the use of bisphosphonate medications to prevent such fractures in 1995, the incidence of fractures in treated patients has significantly decreased. The fracture rates reported in cases of metastatic disease and myeloma have been demonstrated in multiple studies to markedly diminish (roughly a 50% reduction in fracture rates in many studies). Although the tumors still metastasize to the bone, the associated bone destruction and consequent pathologic fracture rate is markedly less. The bisphosphonate medications work by interrupting a biochemical pathway required for bone breakdown by osteoclasts, the cells that normally remove bone in the process of bone remodeling. This bone breakdown step is overactivated in the presence of bony metastases, causing bone loss, bone destruction, and ultimately fractures from the weakening of the bone. Thus, the introduction of bisphosphonate medication has been a major advance over the past 20 years, one that had significant impact on the health of those with myeloma and metastatic cancer to the bone by preventing bone destruction, and thereby preventing bone weakening and subsequent fractures.
The economic burden of SREs in patients with bone metastases is substantial. A recent study showed that the estimated lifetime SRE-related cost per patient suffering from metastatic lung cancer was $11,979 USD, and that radiotherapy accounted for the greatest proportion of cost (61%) by SRE type.1 Finding cures and effective treatments for all types of cancer can help reduce the prevalence and costs associated with bone and joint cancer.
Overall cancers metastatic to bone cause significant pain and morbidity—approximately 50% of patients with metastatic cancer of lung, breast, prostate, and kidney develop bony metastases prior to death. Untreated, these metastases can lead to pathological fractures and cause great pain and disability. Thus, the elucidation of the biochemical steps involved in bone destruction and the development of drugs to combat such steps, have been an example of tremendous scientific advancement and achievement in the field of cancer research and treatment.
Soft tissue tumors, which, like bone tumors, are also called sarcomas, are encountered more frequently than bone and joint tumors. These tumors originate in connective or non-glandular tissue, and can develop in any part of the body that contains fat, muscle, nerve, blood vessels, fibrous tissues, and in any deep tissues, including tissues surrounding joints, bones, or deep subcutaneous tissues. More than half of soft tissue sarcomas develop in the arms or legs. About one in five (20%) are found in the abdominal cavity and present with symptoms similar to other abdominal-based health problems. The rest begin in the head and neck area (about 10%), and in and on the chest or abdomen (about 10%).1
There also are a vast number of non-malignant soft tissue neoplasms and tumors such as lipomas. Also typically included are cystic lesions of the deep tissues. The differentiating feature of soft tissue tumors (sarcomas) is that they arise from these connective tissues and do not arise from organs such as kidneys lungs, intestines, breasts, or thyroid glands.
Additional information on soft tissue sarcomas can be found in Weiss and Goldblum’s Enzinger and Weiss’s Soft Tissue Tumors, 5th ed.2
There are multiple soft tissue sarcomas with varying degrees of aggressive behavior, but virtually all have the capacity to metastasize and cause death. Treatment for high-grade soft tissue sarcomas is typically resection (removal) and radiation. Chemotherapy is playing an ever-increasing role, especially in high-grade (fast-growing) and metastatic cases.
The most common types of soft tissue sarcomas are described below.1 Cancer cells are often referred to as differentiated versus undifferentiated. Differentiation describes how much or how little tumor tissue looks like the normal tissue it came from. Well-differentiated cancer cells look more like normal cells and tend to grow and spread more slowly than poorly differentiated or undifferentiated cancer cells. Differentiation is used in tumor grading systems, which are different for each type of cancer.2
Malignant Fibrous Histiocytomas (MFH)/Pleomorphic Sarcomas (PS) Not Otherwise Specified (NOS)
The most commonly encountered soft tissue sarcoma is malignant fibrous histiocytoma, a tumor of the fibrous tissue most often occurring in the arms or legs. The least differentiated of the sarcomas, in many cases it represents a poorly defined, high-grade soft tissue sarcoma that cannot be further defined pathologically (histologically). A recent trend is to classify these poorly differentiated sarcomas as pleomorphic sarcomas or spindle cell sarcomas not otherwise specified, (NOS) rather than the previous designation as malignant fibrous histiocytoma. Poorly differentiated sarcomas typically affect older individuals. Analysis of annual rates of MFH and PS reflect this evolving diagnostic trend.
Liposarcomas
The next most commonly encountered and reported soft tissue sarcoma is liposarcoma, which is a malignant tumor of the fatty (adipose) tissues. This sarcoma also is more common in older persons. There are several subtypes ranging from the low-grade lipoma-like liposarcoma that rarely metastasizes to high-grade pleomorphic liposarcomas and round cell liposarcomas, which have a prognosis similar to malignant fibrous histiocytoma. Liposarcomas can develop anywhere in the body, but they most often develop in the thigh, around the knee, and inside the back of the abdomen. Seen in a wide range of patient ages, liposarcomas occur most frequently in adults between 50 years and 65 years old. Some liposarcomas grow very slowly, whereas others can grow quickly.
Synovial Sarcomas
The third most commonly encountered soft tissue sarcoma is synovial sarcoma, which is more likely to affect younger adults than previously mentioned sarcomas. The most common location is the thigh. Despite the name synovial sarcoma, most do not occur in joints or in the synovium of joints. It tends to occur mostly in young adults, but can also occur in children and in older people. Many of these cases respond very favorably to chemotherapy with significant shrinkage of the tumor, although resection (surgical removal) and radiation remain the cornerstones of current therapy. The prognosis is similar to malignant fibrous histiocytoma and the other high-grade soft tissue sarcomas mentioned above.
Tumors of Muscle Tissue
Leiomyosarcomas
Smooth muscle cells are found in internal organs such as stomach, intestines, blood vessels, or uterus. This muscle tissue gives these organs the ability to contract involuntarily. Leiomyosarcomas are malignant tumors of involuntary muscle tissue. They can occur almost anywhere in the body, but most often are found in the uterus. A second common site is the retroperitoneum (back of the abdomen) and in the internal organs and blood vessels where leiomyomas also arise. Less often, they develop in the deep soft tissues of the legs or arms. They tend to occur in adults, particularly the elderly. Since they often arise from arteries, resection of these tumors frequently requires an immediate vascular reconstruction.
Rhabdomyosarcomas
Skeletal muscles are the voluntary muscles that control and allow movement of arms and legs and other body parts. Rhabdomyosarcomas are malignant tumors of skeletal muscle. These tumors commonly grow in the arms or legs, but they can also begin in the head and neck area and in reproductive and urinary organs, such as the vagina or bladder. Rhabdomyosarcomas are primarily tumors of children. Clinically and behaviorally, they are in a class by themselves. They are treated with aggressive chemotherapy, as well as surgery and/or radiation in many cases. The aggressive treatments often cause permanent life-altering disability, even in survivors. For more information, see the American Cancer Society document "Rhabdomyosarcoma. [37]”
Malignant Peripheral Nerve Sheath Tumors
Malignant schwannomas, neurofibrosarcomas, or neurogenic sarcomas are malignant tumors of the cells that surround a nerve. The currently favored name for these sarcomas is malignant peripheral nerve sheath tumor.
Tumors of Blood Vessels and Lymph Vessels
Angiosarcomas
Malignant tumors can develop either from blood vessels (hemangiosarcomas) or from lymph vessels (lymphangiosarcomas). These tumors often develop in a part of the body that has been exposed to radiation. Angiosarcomas are sometimes seen in the breast after radiation therapy for breast cancer or in the arm on the same side as a breast that has been irradiated or removed by mastectomy. They are difficult to cure as they spread through the bloodstream to other parts of the body and often spread extensively through the local tissues.
Hemangiopericytoma
These are tumors of perivascular tissue (tissue around blood vessels). They most often develop in the legs, pelvis, and retroperitoneum (the back of the abdominal cavity) and are most common in adults. These can be either benign or malignant. They do not often spread to distant sites, but tend to recur where they started, even after surgery, unless widely excised. Following recent research and further histologic, genetic, and clinical evaluations, these have recently been reclassified as one end of the spectrum of malignant solitary fibrous tumors, or possibly identical to malignant solitary fibrous tumors.1
Hemangioendothelioma
This is a less aggressive blood vessel tumor than Hemangiosarcoma, but still considered a low-grade cancer. It usually invades nearby tissues, and sometimes can metastasize to distant parts of the body. It may develop in soft tissues or in internal organs, such as the liver or lungs.
Kaposi Sarcoma
These cancers are composed of cells similar to those lining blood or lymph vessels. In the past, Kaposi’s sarcoma was an uncommon cancer mostly seen in older people with no apparent immune system problems. It is now most common in people with human immunodeficiency virus (HIV) infection and the acquired immunodeficiency syndrome (AIDS), but can also develop in organ transplant patients who are taking medication to suppress their immune system. It is probably related to infection with a virus called human herpesvirus-8 (HHV-8).
Tumors of Fibrous Tissue
Fibrous tissue forms tendons and ligaments and covers bones, muscles and joint capsules, as well as other organs in the body.
Malignant fibrous histiocytoma (MFH)
MFH is found most often in the arms or legs. Less often, it can develop inside the back of the abdomen. This sarcoma is most common in older adults. Although it mostly tends to grow locally, it can spread to distant sites. It is the most commonly diagnosed soft tissue sarcoma, although now these are more often classified as pleomorphic sarcoma, not otherwise specified (NOS), as discussed above.
Fibrosarcoma
Fibrosarcomas are cancers of fibrous tissue. They have a characteristic herringbone cloth pattern when viewed under the microscope. Fibrosarcomas most commonly affect the legs, arms, or trunk. They are most common between the ages of 20 years and 60 years, but can occur at any age, even in infancy.
Dermatofibrosarcoma protuberans (DFSP)
These tumors are slow-growing cancers of the fibrous tissue beneath the skin, usually noted in the trunk or limbs. They invade nearby tissues but rarely metastasize. They primarily affect young adults. Due to their slow, insidious growth; their uncommon occurrence; and their innocuous appearance, diagnosis is often delayed. The local recurrence rate is higher than many sarcomas, and has been reported to be as high as 50% in some studies. While death due to disease is uncommon (<5%), the local recurrences can cause significant local morbidity.
Fibromatosis/Desmoid tumors
Fibromatosis is one of the names given to neoplastic tumors with features in between fibrosarcomas and benign tumors, such as fibromas and superficial fibrous diseases like Dupytren's disease. They tend to grow slowly, but often steadily. These tumors are often referred to as desmoid tumors. Although they do not metastasize, they do form in response to genetic alterations identical to many cancers and can cause great disability and even death. These tumors can invade nearby tissues, causing great havoc and occasionally even death. Some doctors may consider these to be a type of low-grade fibrosarcomas; most, however, regard these as benign but locally aggressive. Certain hormones, particularly estrogen, may increase the growth of some desmoid tumors. Antiestrogen drugs are sometimes useful in treating desmoids that cannot be completely removed by surgery. Radiation therapy plays a role in treatment, especially in unresectable or recurrent cases. There are ongoing chemotherapeutic trials in place with newer agents that interrupt the various biological processes in the growth of these tumors; these hold great promise for future patients. Additional research into the biology and treatment of these and virtually all tumors is clearly indicated.
Tumors of Uncertain Tissue Type
Through microscopic examination and other laboratory tests, doctors can usually find similarities between most sarcomas and certain types of normal soft tissues, thus, allowing them to be classified based on this histologic appearance. However, some sarcomas have not been linked to a specific type of normal soft tissue due to their unique appearance that does not closely resemble any single tissue type.
Malignant mesenchymoma
These very uncommon sarcomas contain areas showing features of at least two types of sarcoma, including fibrosarcomatous tissue per the original description. Since all connective tissue derive from undifferentiated mesenchymal tissues in an embryologic sense, it has been termed Mesenchymoma. The term has fallen out of favor and it is now thought that many cases may be better classified as one of the subtypes of sarcomas based on the tissue type contained within the tumor.3
Alveolar soft-part sarcoma
This rare cancer primarily affects young adults. The legs are the most common location of these tumors. This is one of the most vascular (blood vessels) sarcomas because it induces an extensive network of vessels to grow in and around the tumor. Because of their very slow growth rate, a delay in diagnosis can occur. Unfortunately, it ultimately has a high mortality rate and can lead to death years after diagnosis. The rate of progression can be quite slow; late metastases are common.
Epithelioid sarcoma
This sarcoma often develops in tissues under the skin of the hands, forearms, feet, or lower legs. Adolescents and young adults are often affected. These are often misdiagnosed as infections and chronic infectious ulcers because of their innocuous appearance and uncommon occurrence. This sarcoma has a much higher propensity for lymph node metastasis than most sarcomas, which usually preferentially metastasize to the lung.
Clear cell sarcoma
This rare cancer often develops in tissues of the arms or legs. It recently has been determined to be a variant of malignant melanoma, a type of cancer that develops from pigment-producing skin cells. How cancers with these features develop in parts of the body other than the skin is not known. As a melanoma, it behaves differently than sarcomas. It has a propensity to spread through the lymphatic system. Local recurrence is common; therefore wide resections are required for complete local eradication.
Other Types of Sarcoma
There are other types of soft tissue sarcomas, but they are less commonly encountered and not included in this discussion.
A recently published study, based on the National Cancer Database NCDB of the American College of Surgeons Commission on Cancers, reports the 13-year experience (1998–2010) with 34 of the most commonly encountered soft tissue sarcomas. This report provides a good overview of the US experience with these, including survival curves, the 2- and 5-year survivorship rate, and various demographic data. A current NCDB analysis of soft tissue sarcomas, including demographic data and survivorships are shown in Table 9A.9.1 to Table 9A.9.3. (Reference Table 8A.9.1 PDF [38] CSV [39], Table 8A.9.2 PDF [40] CSV [41], and Table 8A.9.3 PDF [42] CSV [43])
Soft tissue sarcomas account for less than 1% of all cancer cases diagnosed each year, and for a similar proportion of cancer deaths in a given year. Over the past decade, the overall incidence of soft tissue sarcomas showed a 31% increase in new cases diagnosed annually in the NCDB data, a slightly higher rate of increase than found for all the top 73 cancer sites reported. (Reference Table 8A.3.1 PDF [8] CSV [9] and Table 8A.5.2 PDF [13] CSV [14])
In terms of case numbers, the musculoskeletal health burden in the United States from soft tissue sarcomas is three to four times greater than that of bone and joint sarcomas. For the period from 2006 to 2010, the annual average number of soft tissue neoplasms, including the heart, approximated 14,000 cases/year in the SEER database, a number similar to those for Hodgkin lymphoma.1 Estimated new cases for 2014 by the American Cancer Society are 12,000.2 Soft tissue sarcomas come in a wide variety of forms that affect different age groups, but the most frequently encountered soft tissue sarcomas affect older adults. (Reference Table 8A.3.1 PDF [8] CSV [9])
As previously noted, the National Cancer Data Base (NCDB), a joint program of the Commission on Cancer and the American College of Surgeons, maintains the most thorough database on patients diagnosed with soft tissue sarcomas. Although the NCDB was not created to serve as an incidence-based registry, it currently gathers data on approximately 71% of the cancers treated in the United States. It should be noted this percentage varies from year to year based on the participation and reporting by hospitals to this voluntary database.
Over the 18-year period, 1985 to 2003, 86,355 soft tissue sarcomas of the extremities, shoulders ,and pelvic girdles and trunk were reported. This number excludes approximately 32,250 soft tissue sarcomas of the head and neck, thoracic, and abdominal areas; these patients are generally cared for by non-musculoskeletal specialists. Using a 20-year average and assuming 70% of the annual US cases are included in NCDB, more than 5,700 new cases of soft tissue sarcoma would have occurred annually. This compares to the estimated 12,020 cases predicted for 2014 by the American Cancer Society.2
A 2014 report by Corey, Swett, and Ward examined the adult cases reported to the NCDB of soft tissue sarcomas over a 13-year interval (1998–2010). In 2010, 5,070 soft tissue sarcomas were reported to the NCDB. While the numbers of soft tissue sarcomas reported to the NCDB increased by 19% over this 13-year period, the number of bone sarcomas reported to the NCDB increased by only 10.7% during this same time period.3
While soft tissue sarcomas can be found among all ages, the incidence increases after the age of 55 years. Once a person reaches age 85 years or older, the incidence of diagnosis drops sharply. Males are diagnosed with soft tissue sarcomas at a slightly higher age than females. Blacks are diagnosed an average of 10 years earlier than those who are White. (Reference Table 8A.3.1 PDF [8] CSV [9], Table 8A.3.2 PDF [21] CSV [22], and Table 8A.2.1 PDF [19] CSV [20])
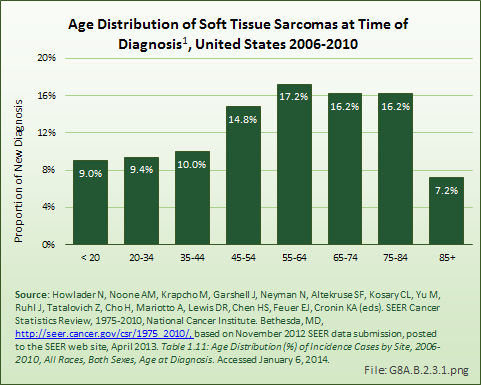
The age distribution at time of death for persons diagnosed with soft tissue sarcomas reflects a relative survival rate that favors younger persons. (Reference Table 8A.3.4 PDF [47] CSV [48])
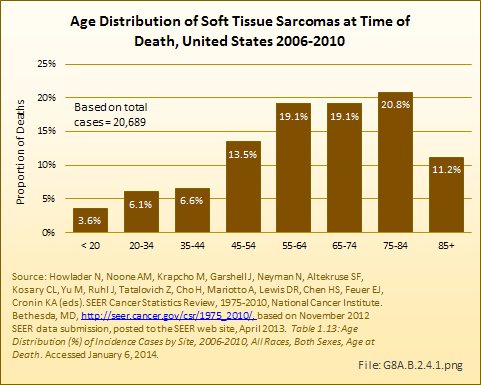
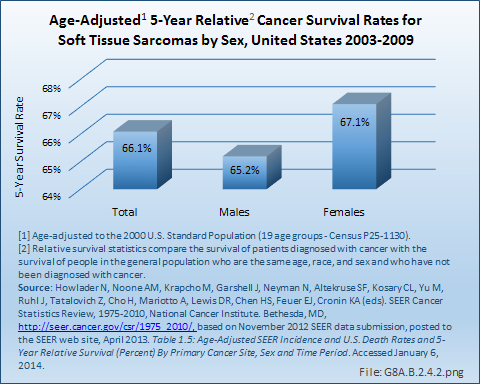
The 5-year survival rate for soft tissue sarcomas is reported at 66% by both the SEER database and the National Cancer Institute (NCI). This is a rate similar to that for bone and joint, uterine/ovarian, and non-Hodgkin lymphoma cancers. Average length of survival after diagnosis is 7 years, similar to that of breast, urinary, and nervous system cancers. White women have a slightly higher 5-year survival rate than do men, and live an average of 1 year longer after diagnosis. However, the reverse is true for Black women, who die an average of 2 years sooner than Black men diagnosed with soft tissue sarcoma. (Reference Table 8A.4.1 PDF [27] CSV [28], Table 8A.4.2 PDF [29] CSV [30], and Table 8A.4.3 PDF [25] CSV [26])
For high-grade soft tissue sarcomas, the most important prognostic factor is the stage at which the tumor is identified. Staging criteria for soft tissue sarcomas are primarily determined by whether the tumor has metastasized or spread elsewhere in the body. Size is highly correlated with risk of metastasis and survival. In general, the prognosis for a soft tissue sarcoma is poorer if the sarcoma is large. As a general rule, high-grade soft tissue sarcomas over 10 cm in diameter have an approximate 50% mortality rate and those over 15 cm in diameter have an approximate 75% mortality rate.
The NCI statistics staging classification of sarcomas is Stage 1, confined to the primary site (localized: 54% of sarcomas are diagnosed at this stage); Stage 2, spread to regional lymph nodes or directly beyond the primary site (regional: 22%); or Stage 3, metastasized (distant: 15%). For the remaining cases, the staging information was unknown. The corresponding 5-year relative survival rates reported are 84% for localized sarcomas, 62% for regional stage sarcomas, 16% for sarcomas with distant spread, and 54% for unstaged sarcomas. The 10-year relative survival rate is only slightly worse for these stages, meaning that most people who survive 5 years are cured.1
Using the staging criteria of soft tissue sarcomas of the American Joint Committee on Cancer (AJCC) produces similar results for sarcomas found in the limbs (arms or legs): 90% 5-year survival rate for Stage 1 sarcomas; 81% for Stage 2; and 56% for Stage 3. Sarcomas identified as Stage 4 have a very low 5-year survival rate. Sarcomas located in other than a limb also have lower survival rates.1
Sarcomas are often staged by orthopedic oncologists with a staging system established by Dr. William Enneking, and adopted and modified by surgical societies primarily consisting of orthopedic oncologists. That may have accounted for the lack of AJCC staging data in many cases of bone and soft tissue sarcomas reported to the NCDB. Nearly 40% of cases for 2000–2011 reported in the NCDB data have an unknown stage. This is a much higher proportion than found among other common cancer types, making it difficult to compare the severity of soft tissue sarcomas to other cancers. However, none of the cases was identified as Stage 1, with the majority at Stage 2 through Stage 4. (Reference Table 8A.5.1 PDF [49] CSV [50])
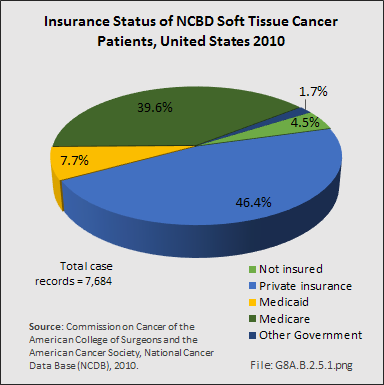
From 1998–2010, information on insurance coverage was available for roughly 96% of patients treated with soft tissue sarcomas. The largest insurance payer was managed care (33%), followed by Medicare with supplement (25%). Private insurance accounted for 14%, while Medicare (11%) accounted for a larger percentile than Medicaid (5%). Detailed NCDB insurance data for 7,684 of 7,878 cases of soft tissue sarcoma reported from the year 2010 showed private insurance paying just over 46%, while Medicare/Medicaid paid a slightly larger share of 47%.1
The total economic costs of malignant soft tissue sarcoma are unknown. Surgery is often the first line of treatment for soft tissue sarcoma. Multiple therapies may be needed later in the course of the patient’s disease, especially in the more advanced cases. In the later stages of the disease in those not cured with surgery alone, significant costs will accumulate as the patients develop pulmonary disease and ultimately die. Hormone therapy, immunotherapy, and bone marrow transplant/endocrine treatments are undertaken in a small number of cases that fail standard treatments. Overall, costs will vary with treatments utilized, number and intensity of treatments, and can easily top $100,000 for a single patient that receives surgery, chemotherapy, and radiation therapy.
Throughout the years 2005–2008, one study reported that the average professional charge for a primary excision was $9,700 and $12, 900 for re-excision. Although every 1-cm increase in size of the tumor results in an increase of $148 for a primary excision, size was not an independent factor affecting re-excision rates. The grade of the tumor was positively associated with professional charge, such that higher-grade tumors resulted in higher charges compared to lower-grade tumors. Analysis including professional technical and indirect charges revealed that, on average, patients undergoing definitive primary excision at their cancer treatment center were charged $40,230. This was compared to $44,770 for to patients receiving definitive re-excision of unsuccessful or incomplete previous resections at the same cancer treatment center. This higher cost did not include the charges and costs generated by their previous unsuccessful or incomplete previous attempt at resection.2
This analysis confirms that proper work-up, evaluation, and treatment are key to maintain costs, as well as, hopefully, improving the outcome for these patients. This cost analysis did not include the costs associated with chemotherapy or radiation therapy, or the costs of diagnostic and follow-up laboratory and radiographic studies, nor the actual costs of care.
The majority of sarcomas develop in people with no known risk factors: There is currently no known way to prevent these cases at this time. Whereas future developments in genomic research may allow genetic testing to identify persons with increased risk to develop soft tissue sarcomas, few such predictors are available at present. Reporting suspicious lumps and growths or unusual symptoms to a doctor, and appropriate evaluation of such abnormalities can help diagnose soft tissue cancer at an earlier stage. Treatment is thought to be more effective when detected early, as smaller-diameter sarcomas have been shown to have improved outcome compared to large sarcomas.
Cancers occasionally spread or metastasize to the soft tissues, such as the muscles and deep tissues of the body, including the thigh and leg. The most likely cancers to do so are cancers of the lung and kidney. As such, this fact must always be borne in mind whenever physicians examine a patient presenting with a new mass in the leg, thigh, or other soft tissues, especially if they have a history of a prior lung or kidney cancer.
Cancers of the musculoskeletal system affect both children and adults, but virtually all tumors have different age-based frequency. Myeloma, the cancer of the bone marrow, affects older persons more, while other bone and joint tumors are more prevalent in children and young adults. Soft tissue sarcomas affect all ages, but most are more common as persons reach middle age and later years. See individual cancer discussions for further information.
Certain primary cancers of bones and joints (osteosarcoma and Ewing sarcoma) are found among those under the age of 30 years in higher proportion than expected for the overall incidence of most sarcomas. In 2006–2010, 43% of bone and joint cancers diagnosed were found in people under the age of 35 years, with more than 27% occurring among children and adolescents under the age of 20. This compares to less than 4% of all cancer sites in people aged 35 years and younger, and only 1% in those younger than 20 years. Hodgkin lymphoma is the only other cancer to affect young people in similar numbers, with a higher percentage of cases diagnosed in the 20-year to 34-year age range. The average age at diagnosis for bone and joint cancers is 42 years, surpassed in youthfulness only by Hodgkin lymphoma, diagnosed at an average age of 38 years. (Reference Table 8A.2.1 PDF [19] CSV [20] and Table 8A.3.2 PDF [21] CSV [22])
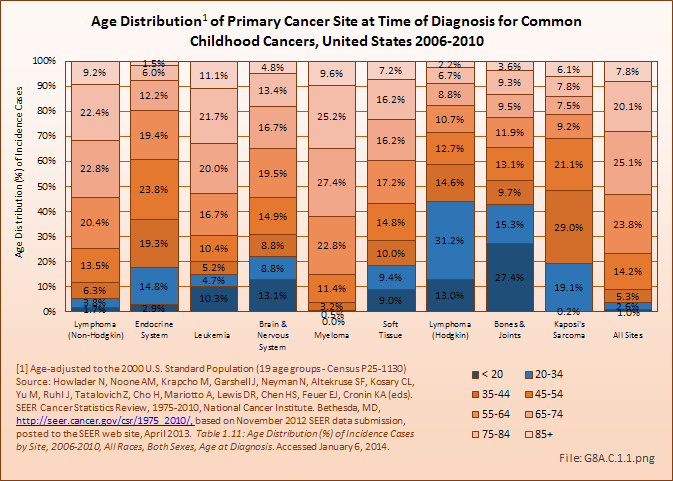
Deaths from bone and joint cancer also are more common in people under the age of 35 years. Between 2006 and 2010, 13% of deaths from bone and joint cancer occurred in children and youth under the age of 20, and an additional 15% among young adults aged 20 years to 34 years. The mortality rate among younger persons from bone and joint cancer comprises only 0.2% of deaths from all types of cancer, but is 8% of cancer deaths in people under the age of 20 years and 5% of deaths among young people aged 20 to 34 years. The relative proportion of deaths from bones and joints cancer was higher in children, youth, and young adults than all other cancer types that disproportionately affect younger people, including brain and nervous system, leukemia, endocrine system, and soft tissue cancers. The average age at death for bone and joint cancer is 59 years, the youngest of all types of cancer. (Reference Table 8A.2.2 PDF [23] CSV [24] and Table 8A.3.4 PDF [47] CSV [48])
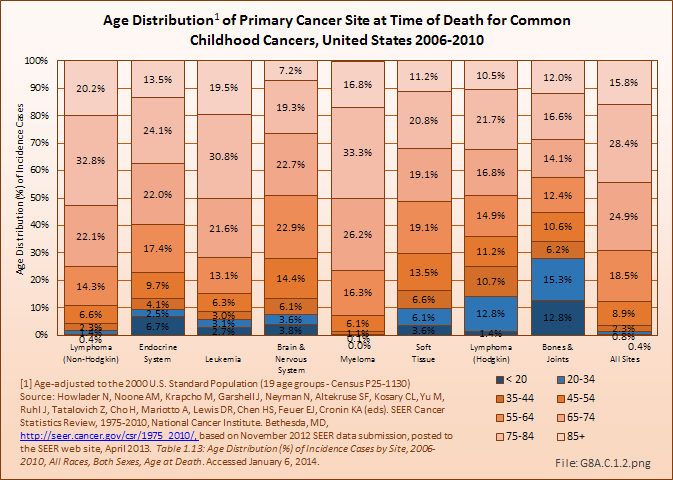
In 2010, osteosarcoma accounted for 54% of the malignant bone tumors in survivors diagnosed with cancer as children and alive on January 1, 2010. The majority of the remaining bone tumors in survivors diagnosed as children and still alive had been diagnosed with Ewing sarcoma (29%). Among the childhood cancer survivors of all ages, 4% were survivors of bone tumors, a proportion that increased slightly with age. Males were a greater proportion of the osteosarcoma survivors than were females until survivors reached middle age, when females were a larger share. Nearly one in four (22%) of the survivors had been diagnosed some 35 years ago. This is comparable with childhood cancer survivors for all types of cancer, where 20% were diagnosed more than 35 years before death. (Reference Table 8A.6.1 PDF [52] CSV [53] and Table 8A.6.2 PDF [54] CSV [55])
Although not considered a childhood cancer, soft tissue sarcomas, which affect all ages, accounted for 9% of new diagnoses in the years 2006 to 2010 in children and young adults under the age of 20. Another 9% were found in the population age 20 to 34. Deaths from soft tissue sarcomas in this time frame were slightly lower, but still accounted for a higher proportion of cancer deaths in the under 35 population (4% and 6%, respectively) than all except bone and joint cancers. (Reference Table 8A.3.2 PDF [21] CSV [22] and Table 8A.3.4 PDF [47] CSV [48])
Rhabdomyosarcoma, a soft tissue sarcoma, accounts for 3% of all new childhood cancers each year. The 5-year survival rate when detected in children under the age of 14 is 64%.1
The high incidence and mortality rate of bone cancers among children, youth, and young adults creates a significant burden on the productivity and life of future generations. Apart from the financial costs, emotional toil, and lost lives from the initial treatments, survivors carry significant functional burdens and continuing care costs. At least 75% of surviving bone and joint cancer patients are treated with limb-salvaging surgery. These surgeries most often require implantation of massive bone-replacing endoprostheses that have limited life span and compromised function, requiring periodic surveillance and revision surgery to repair or replace worn parts. The amputated survivors will require prosthetic limbs, the function of which is clearly limiting in comparison to normal activity. Both procedures are expensive. The cost estimate nearly ten years ago was $25,000 per year for artificial limb replacement of an amputated limb in an active 20- to 30-year- old man in 1997 dollars. The cost estimate was $23,500 for implant, rehabilitation, monitoring, and replacement with limb salvaging endoprostheses.1 More recent cost estimates are not available. Due to chronic pain and overall dysfunction, a large number of such survivors will end up on disability, requiring public support for the majority of their adult lifetime.
More than 60% of myeloma cases are diagnosed in persons age 65 years and older. This is a similar rate to respiratory and urinary system cancers, both of which disproportionately affect older persons. Soft tissue cancers affect all ages, and in relatively equal proportion in the middle years (ages 35 to 64 years) and older population (65 years and older). As previously discussed, bone and joint cancers affect a disproportionate number of younger persons, and are not considered a major cancer of aging. (Reference Table 8A.3.2 PDF [21] CSV [22])
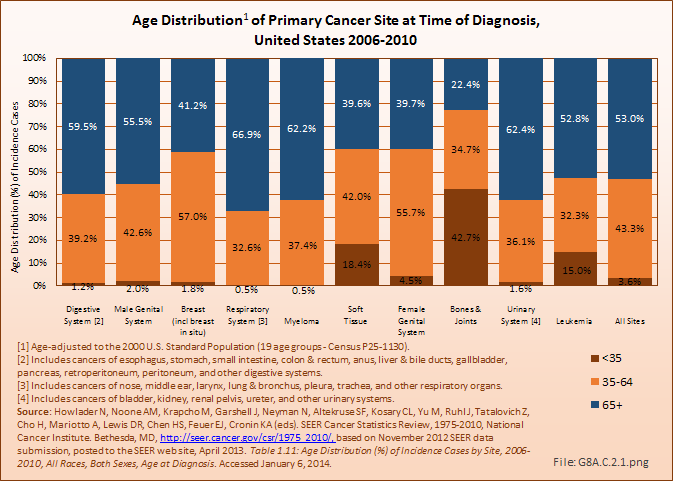
Because of the advanced age of many myeloma patients, and the average survival rate of 6 years following diagnosis coupled with a less than 50% 5-year survival rate, 76% of deaths from myeloma occur to the 65 and older population. Soft tissue cancer deaths occur about equally in people under 65 years and those 65 years and older. Deaths from bone and joint cancers have a lower rate in the 65-year and older population than nearly all other cancers, primarily due to the higher rate of death in those under 35 years of age.(Reference Table 8A.3.4 PDF [47] CSV [48])
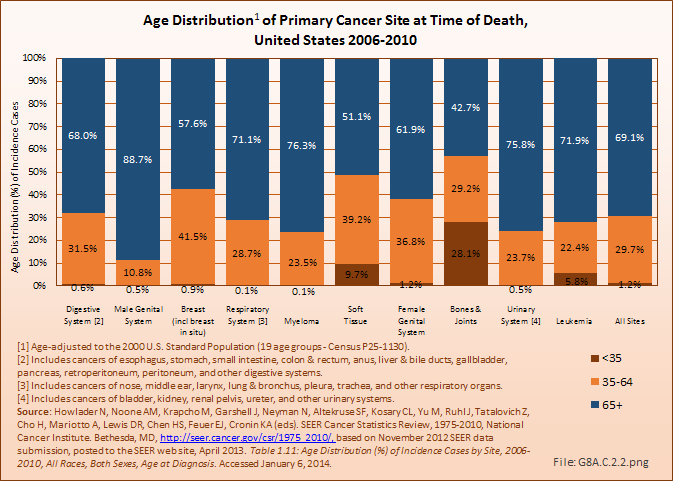
The average age of the population in the United States continues to rise. With age, the human body’s ability to cope with stress and illness declines. As a result, poorer outcomes from cancers are expected in the aged due to greater decline in functional status, adaptability and an increase in co-morbid conditions, all of which have been shown to have an effect on survival.
In addition to the burden of malignant bone and soft tissue tumors, a plethora of benign tumors and tumor-like conditions disable thousands of Americans annually. No national databases on which to base estimates of the prevalence or incidence of such tumors exist.
The relative frequency of more common benign bone tumors has been discerned from prior publications and extrapolation from the primary contributor’s case registry of consecutive surgical cases treated between 1991 through 2004. It should be noted that although Dr. Ward’s personal tumor registry has been updated since 2004, in 2005 additional providers joined his group and began to share care for this cohort of patients. With the resulting tempering in his experience, accurate incidence estimates could no longer be extrapolated from his personal tumor database. Table 9A.7.1 reflects the collected experience reported in the Mayo Clinic publication of 1986, the University of Florida publication from 1983, the J. Mirra experience reported in 1989, and the case series reflecting the practice of Dr. Ward, a full-time solo orthopaedic oncologist in practice from 1991 to 2004, at Wake Forest University Health Sciences in Winston-Salem, NC.
The experience of Dr. Ward during the stated time-period is believed to reflect roughly the general prevalence of bone and soft tissue tumors, since he treated a wide variety of benign and malignant bone tumors in a broad referral practice. All cases in his registry reflected his personally treated patients, ie, none were “consult cases” in which only radiographs or pathology slides were reviewed for outside consulting physicians, such as the Mayo and Mirra series included in their registries. The earlier data sets were accumulated during time periods prior to the full development of the subspecialty of orthopaedic oncology; thus, only the more unusual cases of bone tumors were referred to major medical centers, making estimates of their incidence less reliable. It is believed that, with the exception of bone cysts, general orthopaedic surgeons or other musculoskeletal specialists in North Carolina treated few bone tumors over the period the data was collected, as most were referred to orthopaedic oncologists. Practical experience has confirmed that osteosarcoma is the least likely sarcoma to be treated by anyone other than an orthopaedic oncologist. Dr. Ward and a small group of orthopaedic oncologists treated nearly all patients with an osteosarcoma in North Carolina for the past 22+ years.
As such, comparing the cases of benign bone tumors relative to the cases of osteosarcoma treated by Dr. Ward provides a relative index useful in generating a broad estimate of the prevalence of these benign tumors. By comparing this estimate with the national estimate for the annual occurrence of osteosarcoma, the most commonly encountered primary sarcoma of bone, a rough estimate of the incidence and prevalence of these benign bone tumor diseases was calculated. Because the records only included patients treated surgically, incidence and prevalence estimates also include only patients with these disease states that generally require surgical intervention. This selection process likely excludes small benign tumors, thereby artificially lowering the frequency estimates. (Reference Table 8A.7.1 PDF [57] CSV [58])
Osteochondroma
The most commonly encountered benign tumor of bone is osteochondroma, which typically arises near the long ends of bones. Osteochondromas are often painful because of formation of bursae (small fluid-filled sac) overlying the lesion, tenting and irritation of overlying soft tissues, interference with neurovascular function due to tenting of such structures over the osteochondroma surface, and the potential for deformity in the involved and/or adjacent bones. Long-term complications are uncommon except for rare cases of dedifferentiation into a chondrosarcoma. There is no estimate of the number of patients seen with nonoperatively managed osteochondromas due to lack of records. An annual prevalence of >1,500 surgical cases is based on records kept by Dr. Ward; this is believed to be underestimated. Not included in this estimate are cases treated by general orthopaedic surgeons and pediatric orthopaedic surgeons, who, in addition to orthopaedic oncologists, provide surgical treatment of osteochondromas.
Unicameral Bone Cysts
Unicarmeral bone cysts are the second most commonly encountered benign bone lesions, with an estimated annual prevalence of more than 1,250 surgical cases. The etiology of these fluid-filled bone cysts, usually found in the growing ends of children’s long bones such as the proximal humerus or femur, is unclear. Because they never metastasize and are usually quite characteristic on radiographs, many of these are treated by other orthopaedic surgeons, especially pediatric orthopaedic surgeons. The true incidence, therefore, is probably significantly higher than that estimated by extrapolation from Dr. Ward’s practice experience. These cystic lesions cause weakening of the bone and the patients may require multiple surgeries to rebuild the bone with bone grafts, injections, and other techniques. They occur in children, and typically recur multiple times until skeletal maturity is achieved.
Giant Cell Tumor of Bone
Giant cell tumor of bone, with an estimated annual prevalence of more than 750 cases, is the third most commonly encountered benign bone neoplasm, and accounts for significant disability and dysfunction. This typically occurs near the end of the long bones, most commonly the lower femur or upper tibia, and causes destruction of the bone. The tumor may extend through the cortex of the bone into the soft tissues and, if large enough prior to treatment, can be associated with pathologic fracture of the involved bone. Smaller tumors can be treated with bone resection and reconstruction with bone grafts or cement filler. Cases that are more complicated require sophisticated reconstruction with massive joint replacements and/or massive allografts, and can cause severe long-term disability. On rare occasions, giant cell tumors metastasize to the lungs. In such cases, they typically respond poorly to chemotherapy and may cause death. These tumors are rarely treated by general orthopaedic surgeons. Although currently not considered the standard of care, many patients’ tumors have had excellent responses to denosumab treatment, a monoclonal antibody directed against RANK ligand, the activator of osteoclasts (which are giant cells). This is very similar to the mechanism of action of bisphosphonates. Initial studies with denosumab have shown a very favorable response in the majority of tumors so treated, but presently, even with denosumab pretreatment, surgical resection appears to be ultimately required. With enhanced understanding of the underlying pathogenic mechanisms, non-surgical management may become possible in the near future.
Enchondroma
A fourth commonly encountered tumor that may require surgery is enchondroma, estimated at more than 725 annual surgical cases. Bones typically form as cartilage during the embryo stage of human development, and this cartilage model ultimately converts into bone structure. The cartilage-based growth plates add length to the bones from bone growth. Enchondromas are tumors derived from remnants of these cartilaginous tissues that abnormally remain in the skeleton as remnants or nodules from the normal pattern of maturation and development. If these achieve sufficient size, they can cause cortical bone erosion and pain or fracture, and may present diagnostic challenges requiring biopsy. They often require treatment by curettage and bone grafting. These lesions can dedifferentiate into malignant cartilage tumors called chondrosarcomas. Many small enchondromas are seen incidentally, cause no symptoms, and are treated with simple observation; thus, total incidence of enchondromas is much higher than shown in the surgical data. In addition, the burden of enchondromas requiring surgical treatment is very conservatively estimated, as many are treated by general orthopaedic surgeons.
Other Benign Bone Tumors
Multiple other benign tumors are commonly encountered.
As with the benign bone tumors, there is no national registry of benign soft tissue tumors. By comparing Dr. Ward’s 13 years of practice history from 1991 to 2004, and computing an incidence index relative to that of osteosarcoma, some estimate of the prevalence of surgically treated lesions may be obtained. From this index estimate, a baseline estimate of the national incidence can be calculated. However, benign soft tissue tumors are the most likely category of tumors to be treated by other surgeons, such as general orthopaedic surgeons and general surgeons; therefore, this national estimate is extremely conservative. The prevalence and burden in the United States from benign soft tissue tumors is significantly higher than estimated herein. (Reference Table 8A.7.2 PDF [59] CSV [60])
Detection of the majority of benign soft tissue tumors is from local growth, and may require resection. Benign lesions rarely cause death, and it is rare that an amputation is necessary. However, depending on the site of involvement and size of the lesion, significant disability of the involved extremity and/or joint can occur. The true cost of these otherwise benign neoplasms can be high in terms of healthcare costs, lost work time, morbidity, emotional cost, and disability expenses.
Tumors of Fat Tissue
Lipomas: Benign tumors of fat tissue
Lipomas are the most common benign soft tissue tumor. Most are found under the skin, but they can develop anywhere in the body. Many lipomas are present for years and inactive, but those that are growing lesions and are probably the most commonly resected soft tissue benign tumor. Resection of growing lesions is usually performed in local community settings by multiple surgical specialists and even by primary care practitioners. Only patients with larger and more concerning lesions are typically referred to surgeons with a focus in surgical oncology. Not infrequently, a slow-growing sarcoma is mistakenly diagnosed as a lipoma, leading to a delay in the diagnosis of soft tissue sarcoma. This misdiagnosis can lead to suboptimal resection of the unappreciated sarcoma by the unsuspecting community surgeon.
Lipoblastomas
Lipoblastomas are benign fat tumors that occur in infants and young children.
Hibernomas
Hibernomas are benign fat tissue tumors that behave similarly to lipomas. They are so named because of their brownish coloration that resembles the appearance of the fatty tissue of bears, hence the name hibernomas. They are much less common than lipomas.
Tumors of Muscle Tissue
Smooth muscle benign tumors
Smooth muscle, found in internal organs such as stomach, intestines, blood vessels, or uterus, involuntarily contracts. Leiomyomas are benign tumors of smooth, or involuntary, muscle. Leiomyomas can arise almost anywhere in the body in either men or women because they start in tissues as widespread, for example, as blood vessels or intestine. The most common of these is the fibroid tumor that often develops in the uterus. It is really a leiomyoma (smooth muscle tumor) of the uterus.
Skeletal muscle benign tumors
Skeletal muscle is the muscle that allows movement of arms and legs and other body parts. These are voluntary muscles. Rhabdomyomas are benign tumors of skeletal muscle and are very rare.
Benign Tumors of Peripheral Nerve Tissue (Benign Peripheral Nerve Sheath Tumors)
Neurofibromas, schwannomas (neurilemmomas), and neuromas are benign tumors of nerves. These tumors can occur almost anywhere in the body. An inherited condition called neurofibromatosis, or von Recklinghausen disease, causes people to develop many neurofibromas throughout their body. Some of these may dedifferentiate and become malignant. These dedifferentiated malignant tumors usually form from large neurofibromas in the upper arms, neck, pelvis, or thigh. Patients with the dedifferentiated neural sarcomas have a very poor prognosis since the vast majority of patients ultimately succumb to the cancer.
Tumors of Joint Tissue
Joints are surrounded by tough tissue called synovium, which produces the fluid that lubricates the joint surfaces so they move smoothly. Joint tissue tumors typically arise from the synovium.
Pigmented villonodular synovitis (PVNS) is a benign tumor of joint tissue. It is most common in its nodular form in the hands, and is more common in women than in men. The nodular form rarely recurs following adequate and complete excision. PVNS also occurs in a diffuse form that typically will involve the entire joint lining and has a high recurrence rate after attempted resections. PVNS in its diffuse form is most commonly encountered in the knee joint, where it often causes recurrent bloody effusions (swollen knees filled with bloody fluid).
Tumors of Blood Vessels and Lymph Vessels
Hemangiomas are benign tumors of blood vessels. They are rather common, are often present at birth, and can affect the skin or internal organs. They sometimes disappear without treatment, but when located in muscles and other deep tissues, can be quite problematic and require surgical treatment.
Glomus tumors are benign perivascular (surrounding blood vessels) tumors. They usually are found under the skin and often under fingernails. They are usually small (<1 cm), but are exquisitely tender and painful. They may make the overlying skin sensitive to even light touch from clothing.
Hemangiopericytoma is a tumor of perivascular tissue. It most often develops in the legs, pelvis, and retroperitoneum (the back of the abdominal cavity) and is most common in adults. These can be either benign or malignant. They rarely spread to distant sites, but tend to recur locally following surgical resection unless very widely excised. They may be multifocal.
Lymphangiomas are benign lymph vessel tumors that are usually present at birth. Lymph is a type of fluid that circulates in every tissue of the body. Lymph fluid is collected and routed back into the venous system by the lymphatic system, and contains waste products from tissues and immune system cells. Lymphangiosarcomas are the malignant lymph vessel equivalents of angiosarcomas.
Tumors of Fibrous Tissue
Fibrous tissue forms tendons and ligaments and covers bones as well as other organs in the body.
Fibromas, elastofibromas, extra-abdominal fibromatosis, and fibrous histiocytomas are all benign soft tissue tumors. Fibromatosis is the most problematic of these tumors. They frequently recur following resection, and may require additional treatment with repeat surgery, radiation therapy, chemotherapy, or other therapies. Although these tumors do not metastasize, they can be challenging. Fibromatoses (desmoid tumors) were discussed at length under the malignant soft tissue section above where they are often grouped due to their aggressive nature.
Tumors of Uncertain Tissue Type
Through microscopic examination and other laboratory tests, doctors can usually find similarities between most soft tissue tumors and certain types of normal soft tissues. This is how soft tissue tumors are classified. However, some soft tissue tumors have not been linked to a specific type of normal soft tissue.
Myxoma is a benign tumor that usually is located in muscles but does not develop from muscle cells. The cells of a myxoma produce mucus-like material, a distinguishing feature of this tumor. They are usually found in adults, and rarely recur after treatment. Myxoma must be differentiated from myxofibrosarcoma, a malignant neoplasm that can appear very similar under the microscope as well as in gross appearance. The challenge for the treating physician is to avoid overtreating myxomas and undertreating myxofibrosarcomas, in terms of the extent of normal tissue margin around the tumor to be resected with the tumor to minimize the risk of recurrence.
Granular cell tumors are usually benign tumors of adults that often occur in the tongue, but can be found almost anywhere in the body. They are frequently multifocal.
Tumor-like Conditions of Soft Tissue
Some conditions of soft tissues are caused by inflammation or injury that forms a mass similar to a soft tissue tumor. Unlike a true tumor, they do not come from a single abnormal cell; they have limited capacity to grow or spread to nearby tissues, and never spread through the bloodstream or lymph system. Examples include nodular fasciitis and myositis ossificans, which involve tissues under the skin and muscle tissues, respectively.1
There can also be deposition tumors. The most commonly encountered ones are tophi, often seen in cases of poorly controlled gout. These tumors can achieve massive size, may be mistaken for true tumors, and erode through the skin, causing skin breakdown and infection that may require surgical treatment and antibiotics. Calcium deposits are often seen in renal failure and in poorly managed dialysis patients. They may be painful and may require difficult resection of deposits infiltrated into normal tissue. When not associated with diseases of abnormal calcium metabolism, such as renal failure and dialysis, the disease is termed tumoral calcinosis. It behaves essentially the same as the calcium deposition mentioned in association with renal disease. Amyloid deposition can rarely cause a soft tissue (or bony) mass. These are most often seen in poorly controlled dialysis patients. Rheumatoid nodules are soft tissue deposits of antibody-laden, inflammatory soft tissue masses that can be quite painful and may require resection for symptomatic relief. Adequate treatment of the underlying rheumatoid arthritis with current disease-modifying medications usually prevents the occurrence of such lesions.
Unlike other content areas in this document, the incidence and burden of musculoskeletal tumors relies on the extensive cancer reports available from national cancer databases. ICD-9-CM codes for tumors are presented, but the national databases used in other sections were not analyzed for tumors.
DATA SOURCES
SEER Cancer Statistics Review
Howlader N, Noone AM, Krapcho M, Garshell J, Neyman N, Altekruse SF, Kosary CL, Yu M, Ruhl J, Tatalovich Z, Cho H, Mariotto A, Lewis DR, Chen HS, Feuer EJ, Cronin KA (eds): SEER Cancer Statistics Review, 1975–2010, National Cancer Institute. Bethesda, MD. Available at: http://seer.cancer.gov/csr/1975_2010/ [62]. Based on November 2012 SEER data submission, posted to the SEER web site, April 2013. Accessed February 12, 2015.
National Cancer Database Benchmark Reports, American College of Surgeons
American College of Surgeons: NCDB Benchmarks. National Cancer Database Comparison Reports: NCDB Analytic Cases: Disease Site by American Joint Committee on Cancer Stage, Dx Years: 2000 to 2011. Available at: https://cromwell.facs.org/Bmarks/BMCmp/ver10/Docs/ [63] Accessed February 12, 2015.
American College of Surgeons National Cancer Database
The NCDB is a joint project of the Commission on Cancer of the American College of Surgeons and the American Cancer Society. The data used in this study and this report are derived from a de-identified NCDB file. The American College of Surgeons and the Commission on Cancer have not verified and are not responsible for the analytic or statistical methodology employed, or the conclusions drawn from these data by the investigator and authors of this work.
Case Series, William G. Ward, MD
Case series reflecting the practice of William G. Ward, a full-time solo orthopaedic oncologist in practice from 1991 to 2004, at Wake Forest University Health Sciences in Winston-Salem, NC.
ICD-9-CM Codes
MALIGNANT NEOPLASM OF BONE AND ARTICULAR CARTILAGE
Malignant neoplasm of bone and articular cartilage: 170
Malignant neoplasm of bones of skull and face except mandible: 170.0
Malignant neoplasm of mandible: 170.1
Malignant neoplasm of vertebral column excluding sacrum and coccyx: 170.2
Malignant neoplasm of ribs sternum and clavicle: 170.3
Malignant neoplasm of scapula and long bones of upper limb: 170.4
Malignant neoplasm of short bones of upper limb: 170.5
Malignant neoplasm of pelvic bones sacrum and coccyx: 170.6
Malignant neoplasm of long bones of lower limb: 170.7
Malignant neoplasm of short bones of lower limb: 170.8
Site unspecified: 170.9
Malignant neoplasm of connective and other soft tissue: 171
Of head face and neck: 171.1
Of upper limb including shoulder: 171.2
Of lower limb including hip: 171.3
Of thorax: 171.4
Of abdomen: 171.5
Of pelvis: 171.6
Of trunk unspecified: 171.7
Malignant neoplasm of other specified sites of connective and other soft tissue: 171.8
Site unspecified: 171.9
BENIGN TUMORS
Benign neoplasm of bone and articular cartilage: 213
Other benign neoplasm of connective and other soft tissue: 215
Links:
[1] https://www.facs.org/quality%20programs/cancer/ncdb
[2] http://seer.cancer.gov/
[3] http://surveillance.cancer.gov/statistics/types/survival.html
[4] http://seer.cancer.gov/seerstat/WebHelp/Relative_Survival.htm
[5] http://seer.cancer.gov/statistics/types.html
[6] https://bmus.latticegroup.com/docs/T8A.1.1.pdf
[7] https://bmus.latticegroup.com/docs/T8A.1.1.csv
[8] https://bmus.latticegroup.com/docs/T8A.3.1.pdf
[9] https://bmus.latticegroup.com/docs/T8A.3.1.csv
[10] https://bmus.latticegroup.com/docs/T8A.3.3.pdf
[11] https://bmus.latticegroup.com/docs/T8A.3.3.csv
[12] http://seer.cancer.gov/seerstat/
[13] https://bmus.latticegroup.com/docs/T8A.5.2.pdf
[14] https://bmus.latticegroup.com/docs/T8A.5.2.csv
[15] http://seer.cancer.gov/statfacts/html/bones.html
[16] http://seer.cancer.gov/statfacts/html/mulmy.html
[17] https://bmus.latticegroup.com/docs/T8A.1.2.pdf
[18] https://bmus.latticegroup.com/docs/T8A.1.2.csv
[19] https://bmus.latticegroup.com/docs/T8A.2.1.pdf
[20] https://bmus.latticegroup.com/docs/T8A.2.1.csv
[21] https://bmus.latticegroup.com/docs/T8A.3.2.pdf
[22] https://bmus.latticegroup.com/docs/T8A.3.2.csv
[23] https://bmus.latticegroup.com/docs/T8A.2.2.pdf
[24] https://bmus.latticegroup.com/docs/T8A.2.2.csv
[25] https://bmus.latticegroup.com/docs/T8A.4.3.pdf
[26] https://bmus.latticegroup.com/docs/T8A.4.3.csv
[27] https://bmus.latticegroup.com/docs/T8A.4.1.pdf
[28] https://bmus.latticegroup.com/docs/T8A.4.1.csv
[29] https://bmus.latticegroup.com/docs/T8A.4.2.pdf
[30] https://bmus.latticegroup.com/docs/T8A.4.2.csv
[31] https://bmus.latticegroup.com/docs/T8A.8.1.pdf
[32] https://bmus.latticegroup.com/docs/T8A.8.1.csv
[33] http://www.cancer.net/cancer-types/osteosarcoma-childhood/statistics
[34] http://www.cancer.net/cancer-types/ewing-sarcoma-childhood/statistics
[35] http://seer.cancer.gov/statfacts/more.html.
[36] http://www.cancer.org/cancer/sarcoma-adultsofttissuecancer/detailedguide/sarcoma-adult-soft-tissue-cancer-soft-tissue-sarcoma
[37] http://www.cancer.org/cancer/rhabdomyosarcoma/detailedguide/rhabdomyosarcoma-detailed-guide-toc
[38] https://bmus.latticegroup.com/docs/T8A.9.1.pdf
[39] https://bmus.latticegroup.com/docs/T8A.9.1.csv
[40] https://bmus.latticegroup.com/docs/T8A.9.2.pdf
[41] https://bmus.latticegroup.com/docs/T8A.9.2.csv
[42] https://bmus.latticegroup.com/docs/T8A.9.3.pdf
[43] https://bmus.latticegroup.com/docs/T8A.9.3.csv
[44] http://www.cancer.gov/dictionary?cdrid=46445
[45] http://www.facs.org/cancer/ncdb/publicaccess.html
[46] http://www.cancer.org/cancer/sarcoma-adultsofttissuecancer/detailedguide/sarcoma-adult-soft-tissue-cancer-key-statistics
[47] https://bmus.latticegroup.com/docs/T8A.3.4.pdf
[48] https://bmus.latticegroup.com/docs/T8A.3.4.csv
[49] https://bmus.latticegroup.com/docs/T8A.5.1.pdf
[50] https://bmus.latticegroup.com/docs/T8A.5.1.csv
[51] http://www.cancer.org/cancer/sarcoma-adultsofttissuecancer/detailedguide/sarcoma-adult-soft-tissue-cancer-staging
[52] https://bmus.latticegroup.com/docs/T8A.6.1.pdf
[53] https://bmus.latticegroup.com/docs/T8A.6.1.csv
[54] https://bmus.latticegroup.com/docs/T8A.6.2.pdf
[55] https://bmus.latticegroup.com/docs/T8A.6.2.csv
[56] http://www.cancer.net/cancer-types/rhabdomyosarcoma-childhood/statistics
[57] https://bmus.latticegroup.com/docs/T8A.7.1.pdf
[58] https://bmus.latticegroup.com/docs/T8A.7.1.csv
[59] https://bmus.latticegroup.com/docs/T8A.7.2.pdf
[60] https://bmus.latticegroup.com/docs/T8A.7.2.csv
[61] http://www.cancer.org/cancer/sarcoma-adultsofttissuecancer/detailed guide/index
[62] http://seer.cancer.gov/csr/1975_2010/
[63] https://cromwell.facs.org/Bmarks/BMCmp/ver10/Docs/