

Osteoporosis is a chronic musculoskeletal condition characterized by reductions in bone mass and quality accompanied by microarchitectural changes that lead to reduced bone strength. Reductions in bone mass, quality and strength increase the risk for fragility fractures. The primary diagnostic test for osteoporosis is measurement of bone mineral density (BMD) using dual energy X-ray absorptiometry (DXA). DXA testing provides an estimate of areal BMD in g/cm2,1 and the estimate is converted into a T-score by comparing it to the distribution of BMD levels of young adults. Using thresholds developed by the World Health Organization, osteoporosis is defined as a T-score ≤ -2.5. T-scores between -2.5 and -1.0 identify individuals with low bone mass (osteopenia) and T-scores ≥ -1.0 represent normal bone mass.2 It should be noted that older persons who sustain a hip fracture and/or a vertebral (spine) fracture are considered to have osteoporosis even in the absence of undergoing BMD measurement.
In the United States, the national prevalence of osteoporosis based on BMD data comes from the National Health and Nutrition Examination Survey (NHANES). NHANES is conducted by the National Center for Health Statistics, Centers for Disease Control and Prevention (CDC), to assess the health and nutrition status of a representative sample of the noninstitutionalized US population. Participant interviews are conducted in their homes to assess a variety of health states. They receive standardized physical measurements, including BMD measurements via DXA, in mobile examination centers that are moved around the nation. In 2017, the most recent national estimates of the prevalence of osteoporosis based on femoral neck and lumbar spine BMD data were released using NHANES data from the 2005–2006 through the 2013-2014 cycles (NHANES data is collected in 2-year cycles).1 In addition to reporting the prevalence estimates overall and by sex, this report expanded by race and ethnicity results to include non-Hispanic Asians.
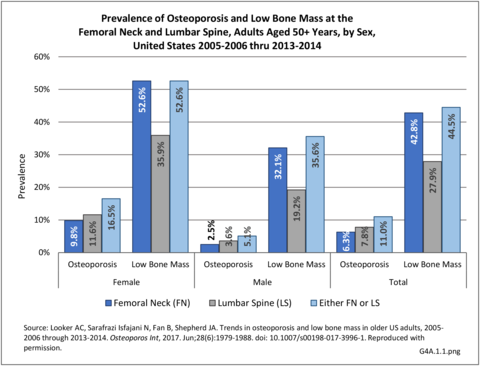
Using the 2005-2006 thru 2013-2014 data, the average overall prevalence of osteoporosis in US adults aged 50 and over was 11.0%, or roughly 12 million adults, when using either the femoral neck or lumbar spine T-score. The prevalence was significantly higher in women (16.5%) than men (5.1%). The overall prevalence of low bone mass was 44.5%, representing ~45 million adults. The prevalence of low bone mass was higher in women (52.6%) than in men (35.6%).1 (Reference Table 4A.1.1 PDF CSV)
Among women, non-Hispanic Asians over the age of 50 years had the highest prevalence of osteoporosis (40.0%), followed by Hispanic women (20.5%), non-Hispanic white women (17.0%), and non-Hispanic black women (8.2%). Among men, non-Hispanic Asian men had the highest age-adjusted prevalence of osteoporosis (7.5%), followed by non-Hispanic whites (6.0%), Hispanics (5.9%), and non-Hispanic black men (1.9%). Hispanic (57.0%) and non-Hispanic white (54.6%) women had the highest age-adjusted prevalence of low bone mass, followed by non-Hispanic Asian (47.0%) and non-Hispanic black (40.4%) women. Asian (47.7%) and Hispanic (38.1%) men had the highest age-adjusted prevalence of low bone mass, followed by non-Hispanic white (37.3%) and non-Hispanic black (25.7%) men.1 (Reference Table 4A.1.2 PDF CSV)
The Hispanic population includes populations based on country of origin, including persons of Cuban, Mexican, Puerto Rican, South or Central American, or other Spanish culture or origin regardless of race. Previous studies have shown different fracture rates between Mexican, Cuban, and Puerto Rican American men and women,2 and it is likely that the prevalence of osteoporosis and low bone mass are different between Hispanic ethnic subgroups. A recent analysis utilizing data from the Boston Puerto Rican Osteoporosis Study (BPROS) compared the prevalence of osteoporosis and low bone mass by race/ethnic distribution in the US population as reported in the 2005-2010 NHANES to that of the prevalence estimates in their age and sex cohorts.3 They found that Mexican American women had the highest prevalence of osteoporosis at the femoral neck or spine (16%), followed by Puerto Rican women (10.7%), non-Hispanic white women (10.1%), and then non-Hispanic black women (3.8%). However, among men, Puerto Rican men had the highest prevalence of osteoporosis (8.6%), followed by Mexican American men (3.9%), non-Hispanic white men (2.3%), and non-Hispanic black men (1.3%), highlighting the burden of bone health in multiple Hispanic communities. The heterogeneity in osteoporosis prevalence observed by Hispanic origin likely applies to Asian groups as well, as indicated by age-standardized hip fracture incidence rates by country of origin. These data showed that women from Taiwan had higher hip fracture incidence rates than women from China.4 (Reference Table 4A.1.3 PDF CSV)
In addition to BMD testing, osteoporosis can be clinically diagnosed in individuals who have had a fragility fracture, irrespective of BMD, particularly if that fracture occurs at the hip or spine.1
There is no single national database that captures all hip and clinical spine fractures and estimates how many additional people have fractures without BMD-defined osteoporosis. One of the most comprehensive databases for evaluation of the total number of hospitalized fractures is the Agency for Healthcare Research and Quality’s (AHRQ) Nationwide Inpatient Sample (NIS). The NIS includes more than 8 million inpatient hospitalizations each year from all payers in the United States.
We evaluated the 2013-2014 NIS sample to estimate the number of discharges for fragility fractures, defined as a fracture of the hip, spine, pelvis, femur, humerus or wrist. Among US adults aged 50 and above, there were a total of 19.5 million hospital discharges, of which 540,600 (2.8%) were for fragility fractures. The distribution of these fractures by fracture type, sex, and age can be seen below. As expected, the prevalence of fractures is higher in women than in men, with fragility fractures representing 3.7% of the total hospital discharges in 2013-2014 in women, compared to only 1.8% in men. Likewise, the prevalence of fracture increased with age, with persons age 80 and over representing only 11% of the 50+ population, but accounting for 25% of fractures. (Reference Table 4A.2.1 PDF CSV and Table 4A.2.2 PDF CSV)
We also evaluated the distribution of fragility fractures by race and ethnicity. Overall non-Hispanic white individuals had the highest number of hospital discharges associated with fragility fractures, particularly at the hip and the spine. Hispanic individuals had a larger number of discharges for fragility fractures other than hip and femur than the non-Hispanic black and the other race and ethnic groups. However, when compared to the proportion of the over 50 population in the US by race/ethnicity group, non-Hispanic whites, with a ratio of 1.1 (fractures to population) accounted for the highest incidence of all fractures. Non-Hispanic blacks had the lowest ratio (0.4), followed by Hispanics (0.6), and non-Hispanic others (0.7). (Reference Table 4A.2.3 PDF CSV)
Relying on hospitalized fractures underestimates the true prevalence of all fragility fractures in the United States. For example, wrist fractures do not require hospital treatment and approximately two-thirds of all vertebral fractures are not clinically diagnosed.2 This is evident in our data. The 2012-2014 NIS dataset reports over 500,000 hospital discharges for fractures. Annually, there are about 1.5-2 million fractures in the US,3, thus, relying on the inpatient database alone clearly underestimates fracture prevalence. The data previously shown also portrays a low number of hospital discharges observed for fracture sites like wrist and spine, which are more frequently observed fracture sites. Again, as previously mentioned, these fracture types do not always require an inpatient hospital stay.
To account for the fracture prevalence underestimation using inpatient data, particularly for wrist fractures, we also utilized the HCUP National Emergency Department Sample (NEDS) to evaluate fragility fractures. The NEDS produces national estimates about ED visits across the country, regardless of whether they result in a hospital admission.4 The 2013-2014 NEDS data included a total of 46.7 million ED visits for all causes, with a primary diagnosis of a fragility fracture in 935,700 (0.9%) of the visits. Again, more visits related to fragility fractures were recorded for women (1.2%) than in men (0.5%). Likewise, the proportion of the total visits for fragility fractures increased from 1.1% to 5.9% hospital discharges age 50-59 years to age 80 years and older, and 0.9% to 4.3% emergency visits for the same age brackets. (Reference Table 4A.2.2 PDF CSV)
The figure below shows the distribution of the fragility fractures by fracture type overall, by sex, and by age. Unlike the hospital discharge data, there are more emergency department visits for fragility fractures of the wrist and humerus. Although overall there is an increase in ED visits for fragility fractures by age, the pattern is not consistent for all types of fractures. This may be due to changes in fracture risk and severity by age. The NEDS data does not routinely collect information on race/and ethnicity, so we were unable to evaluate the distribution of the fractures by race and ethnicity.
Given the clear difference in distribution of hospital discharges and ED visits for fragility fractures such as wrist fractures, the use of multiple data sources to estimate fracture incidence and prevalence in the US seems optimal. However, there are still situations where some fractures are identified via imaging and not treated (e.g. spine fractures), treated in a physician’s office, or are not the primary diagnosis of an ED or inpatient visit but are still a cause of admission. Algorithms can capture these and other situations to identify fragility fractures in administrative claims data, particularly for fragility fractures related to osteoporosis (hip, pelvis, femur, tibia/fibula or ankle, radius/ulna, humerus or scapula, clavicle, and clinical vertebral). Such an algorithm has been validated against medical records in the Medicare patient population and has been shown to have high positive predictive values overall and by each of their definitions.5
The 2015 incidence of the six major osteoporosis fractures in the Medicare 5% Sample can be found in the Figure below. A total of 465,820 fractures were identified, with 75% of the fractures occurring in women. As observed in the NIS data, hip and spine fractures were the most predominant fracture types, with incidences of 100.0 and 105.9 per 1,000 beneficiaries in women and 41.3 and 42.7 per 1000 in men with a fracture diagnosis, respectively. (Reference Table 4A.2.6 PDF CSV)
Using the 5% Medicare inpatient claims database, it was reported that the decline in hip fractures observed in women had plateaued since 2012.6 We also evaluated the trends in hip fracture in the 2010-2014 NIS data. We observed that from 2010 to 2014, there was a 3.5% and 1.4% increase in the number of hip and femur fracture discharges, respectively. All other fracture sites had decreases ranging from 12% to 22%. When evaluating the difference between 2012 and 2014, the inflection point observed in the previously cited paper,6 there were 5.4% and 7.5% increases in hip and femur fractures, respectively. The decline in other fractures ranged from 0.1% to 11%. However, overall, fragility fracture discharges remained within a relatively small range for each of the primary fractures over the five-year period. (Reference Table 4A.2.7 PDF CSV)
We also evaluated trends in hip fracture using Medicare data among women in the 5% sample and all women with postmenopausal osteoporosis (PMO). Women with PMO were identified if they had an osteoporosis diagnosis code, were taking osteoporosis medications, and/or sustained a major osteoporotic fracture.7 Similar to previous reports, we observed a plateau in the incidence of hip fractures in the most recent years of Medicare data available. In the 5% sample, more generalizable to all female Medicare beneficiaries, we observed a 3.1% increase in the age-standardized incidence rate from 2010 to 2015; however, the confidence intervals for these incidence rates overlapped, suggesting no significant difference between the two rates [8.05 (7.87, 8.24) vs. 8.30 (8.09, 8.52)]. The incidence rate from the last four years, 2012 to 2015, showed a slight increase, but again, this difference was not significant [8.42 (8.22, 8.61) vs. 8.30 (8.09,8.52)], indicating a relatively stable hip fracture incidence rate during this time window.
However, in the PMO cohort, a cohort potentially at higher risk for fragility fractures, we observed an increase in the incidence rate of hip fracture over time. From 2010 to 2015, the hip fracture incidence increased by 9.1%, slowing down from 2012 to 2015, to a hip fracture incidence increase of 4.2%. Each of these increases was statistically significant, as the confidence intervals did not overlap during these time windows. This increase in the hip fracture incidence could be due to the osteoporosis “treatment gap,” which will be discussed later in the chapter. (Reference Table 4A.2.8 PDF CSV)
Osteoporosis and fractures are associated with increases in healthcare utilization, including hospitalization stays, physician office visits, and pharmacy use. We used NIS data to evaluate a variety of healthcare utilization characteristics by fracture site and a variety of demographic characteristics.
In 2014, the mean (standard deviation (SD)) length of stay (LOS) for all fragility fractures is 5.3 (0.87) days. The LOS was the highest for femur fractures (6.1 days) and the lowest for wrist fractures (3.6 days). When evaluating by sex, men tended to have longer LOS than women for all fracture sites. (Reference Table 4A.3.1 PDF CSV)
Overall, it appeared that younger individuals had slightly longer LOS than older individuals. This was most apparent for pelvic and femur fractures in comparison to the other fracture sites. The longer LOS could be due to traumatic nature of these types of fracture in younger individuals; however, the level of trauma could not be determined from these data. (Reference Table 4A.3.1 PDF CSV)
Lastly, LOS was significantly longer in non-Hispanic black persons with fractures at all fracture sites compared to the other race and ethnic groups. (Reference Table 4A.3.3 PDF CSV)
Hospital charges are based on standard hospital cost for patient stays and procedures and provide a comparison between groups. They are not indicative of actual cost paid by insurance companies and patients. Charges are associated with individual hospital stays with a fracture diagnosis and do not include additional costs associated with the same fracture for any readmission, physician care, or long-term care required.
Overall, in 2014, fragility fractures were associated with nearly $60,000 in mean hospital charges. The highest charges were associated with femur fractures ($75,000), whereas the lowest charges were associated with pelvic fractures ($39,700). Charges were higher in men with fractures than women. (Reference Table 4A.3.1 PDF CSV)
Charges decreased as age increased for all fracture types. The reason for lower hospital charges among the eldest patients is not fully understood but is possibly due to shorter stays, particularly for spine, pelvic and femur fractures, among those age 80 and over where transfer rates to short/long term care is higher. Elderly patients also may not have surgery for fixation. (Reference Table 4A.3.1 PDF CSV)
Overall and by fracture site, Hispanics had the highest hospital charges, followed by non-Hispanic blacks and people from other race and ethnic groups, and then non-Hispanic Whites. (Reference Table 4A.3.3 PDF CSV)
Despite year-to-year rise and fall in healthcare treatments and visits for osteoporosis, total healthcare visits to physicians, ambulatory nonphysician healthcare sites, and for home health services, along with the number of prescriptions filled, have each remained relatively steady over the past 15 years (2000 to 2014). The general trend was a rising number of visits in the early 2000s, with a decline since about 2007. The exception is home health visits. (Reference Table 8.2.4 PDF CSV)
Medical costs reported in the Economic Cost chapter of this report, on which this section is based, are analyzed using the US Department of Health and Human Services, Agency for Healthcare Research and Quality, Medical Expenditures Panel Survey (MEPS) and capture four types of healthcare resources consumed: ambulatory visits (to both physicians and nonphysicians), prescription medications, home healthcare visits, hospital discharges, plus “residual” (all other types of care). Unlike the hospital charges discussed previously, the MEPS utilizes expenditures actually paid for healthcare by insurance companies and patients.
Overall, ambulatory care visits accounted for the largest share of per-person direct cost for people with an osteoporosis condition along with other conditions. At an average cost of $4,035 per person on average 2012-2014, ambulatory cost of care increased 47% from 1998 to 2014 in 2014 dollars. However, the share of per person total direct cost for osteoporosis ambulatory care remained constant at 31%. The mean per-person cost for inpatient care, after dropping for several years, was slightly higher at $3,381 in 2012-2014 than in 1998-2000 in 2014 dollars. The share of total direct costs for inpatient care dropped by 25% between 1998-2000 and 2012-2014. The greatest change in average per-person cost was for prescriptions, rising from $1,771 in 1998-2000 to $3,494 in 2012-2014, in 2014 dollars, an increase of 97%. The share of cost for prescriptions rose 37%, from 20% to 27%. (Reference Table 8.4.4 PDF CSV)
Total direct per-person healthcare costs in 2012-2014 for people with an osteoporosis condition along with other conditions were $12,869, an increase of 44% since 1998-2000 in 2014 dollars. Incremental direct per-person costs, those costs most likely attributable to an osteoporosis condition and a more useful statistic, cannot be calculated for osteoporosis because of the small sample size. (Reference Table 8.6.4 PDF CSV)
Total aggregate direct costs for all persons were $73.6 billion in 2012-2014, a rise of 118% from the $28.1 billion in 1998-2000, in 2014 dollars. (Reference Table 8.6.4 PDF CSV)
Indirect costs associated with lost wages for people ages 18 to 64 years are not calculated for those with an osteoporosis condition. Osteoporosis is rarely cited as a reason for lost workdays or bed days, in part because of the older age of the most commonly affected adults. However, approximately 50% of people with hip fractures do not regain their prior activity level, leading to societal costs for added care, particularly among caregivers whose income may be reduced due to lost workdays and productivity, as well as physical therapy, transit assistance, and inhome care. Similarly, vertebral compression fractures due to osteoporosis contribute to spinal deformity, reduced mobility, and the need for assistance with activities of daily living, further increasing indirect societal costs.
The Bone and Joint Decade, from 2000 to 2010, saw an increase in the awareness of and education for osteoporosis, as well as therapies used in the clinical management of osteoporosis and fragility fracture. Although cause and effect cannot be determined, over time epidemiologic studies reported a steady decline in fractures, particularly of the hip.1 However, with the emerging information on the serious, yet rare, side effects of long-term exposure to some of the osteoporosis medications (particularly Nitrogen-containing bisphosphonates), changes in care from the physician and patient level emerged in the field, which have led to what is now being called an osteoporosis treatment gap.2
In October 2010, as a result of reports associating long-term use of anti-osteoporosis medications (particularly the Nitrogen-containing bisphosphonates and also denosumab) with rare adverse events such as atypical femoral fractures (AFF) and osteonecrosis of the jaw (ONJ), the FDA issued revised guidance concerning long-term use of bisphosphonate treatments. An AFF starts as a weakening of the outer rim of the femur below the hip area as a tiny crack resembling a stress fracture. Unlike more common osteoporosis fractures, AFF develop over time from normal activities. If warning signs are not addressed, eventually the thigh bone may break completely. ONJ occurs when the jaw bone is exposed. Most cases of ONJ happen in long-term or very high dose bisphosphonate users after a dental extraction. The risk of ONJ in patients taking bisphosphonates for osteoporosis has a rate estimated between .001 and .01%.3 In October 2010, the FDA provided guidelines on patient review for therapies used longer than three to five years, typically bisphosphonates, and suggested taking a break (or "holiday") from potent antiresorptive medications, including Nitrogen-containing bisphosphonates, in appropriate patients.
There is limited evidence to support both the practice of “drug holidays” and to determine the appropriate time to resume therapy, if one chooses to have a “drug holiday.” The use of DXA and bone turnover markers may assist in the clinical decision (e.g., a decline in bone mass density (BMD) greater than the smallest detectable difference in DXA or an increase in bone turnover markers beyond the normal premenopausal range); however, some patients never return to therapy. As previously reported in the Fracture Trends section, since 2012, the decline in hip fractures that was previously observed in older women has plateaued or even reversed in the US. This finding may be associated with an osteoporosis treatment gap that was highlighted earlier. The major key challenge for the future is for researchers to identify factors associated with the treatment gap, and work with patients on the best way to mediate them so physicians can continue to provide the best care for patients in reducing the risk for fractures.
Currently available data are not conducted at the person-level and do not link lifestyle factors or treatment with diagnosis; therefore, causal relationships cannot be established. However, there are many factors that may be associated with the current osteoporosis treatment gap, including (1) a decrease in DXA testing, (2) a decrease in the number of providers performing DXAs, and (3) the decreasing prevalence of women with osteoporosis diagnoses, which may contradict some of the epidemiologic data.
Depicted in the Figure below, the prevalence of DXA testing has decreased in the rate given to female Medicare recipients age 65 and older from a peak of 13.2% in 2008 to 11.3% in 2014. Likewise, the number of DXA providers, particularly office-based providers, has decreased from 22,355 in 2008 to 15,952 in 2014, a loss of 6,403 providers. This decrease was not offset by a slight rise of 518 hospital DXA providers between 2007 to 2014.4
Another potential factor is the decline in the use of medications. Drug holidays have become an option in treatment (see above), and along with the fear of side effects, there has been a decrease in the use of anti-osteoporosis medications. A study using commercial pharmacy data evaluated the number of dispensed prescriptions of both oral and parenteral bisphosphonates from 2002 to 2012. The total number of dispensed prescriptions reached a high of 31.0 million in 2008 and decreased to 14.7 million in 2012.5 Likewise, a study of Medicare patients with a hip fracture showed that the probability of osteoporosis medication use within 12 months of hip fracture discharge decreased from 40% in 2002 to 21% in 2011.6
To potentially understand the decline in medication use, researchers conducted ecologic studies describing medication use over time and evaluating the timing of the various FDA safety announcements. A study using commercial data evaluated the proportion of hip fracture patients using anti-osteoporosis medications.7 They stratified by bisphosphonates and all other osteoporosis medications. The investigators plotted the distribution of users over time and indicated when the FDA released announcements regarding safety around three adverse events: osteonecrosis of the jaw (May 2005), atrial fibrillation (Oct 2007), and atypical femur fractures (Mar 2010). The study, depicted in the graphic below, clearly showed that the FDA announcements may have had temporal associations with the use of bisphosphonates, but not apparently on the use of other osteoporosis medications. Specifically, the authors found a 4% decrease in the odds of bisphosphonate use in hip fracture patients every quarter after the release of the atypical femur fracture announcement, whereas there was no association in the other osteoporosis medications.8
Poor health education is another factor associated with the osteoporosis treatment gap. Although the Bone and Joint Decade increased the amount of osteoporosis education that was being circulated, it might not have reached all racial and ethnic groups. The prevalence of osteoporosis among Hispanic populations is equal to or higher than non-Hispanic whites, although the number of fractures is higher among non-Hispanic whites. Based on US Census data, the Hispanic population is the fastest growing ethnic group in the US overall, as well as among older adults,9,10 suggesting the need for culturally appropriate osteoporosis education information. Affiliates with the National Osteoporosis Foundation (NOF) have developed a Hispanic version of the NOF website to empower Hispanic women about osteoporosis, and “Fit to a T,” one of the patient education tools sponsored by the USBJI also sponsors Spanish language versions of several osteoporosis programs. This is one of the first steps in reducing disparities seen in DXA screening and medication use among Hispanic women.
Additional education is also needed for black women and the providers who serve them. Although black women have a generally lower prevalence of osteoporosis, those who have fractures have worse outcomes than their white counterparts. Several studies have shown that mortality after hip fractures is nearly 30% higher in black women than white women, even after adjusting for demographic, health, and surgical factors. Black women are less likely to be screened for osteoporosis,11, even high risk women who have sustained hip fractures,12 and less likely to receive medications.13,14,15 Although this disparity may have had a minimal impact on the current osteoporosis treatment gap, it still represents an area that needs improvement in the field.
Of three new therapies on the horizon in the last edition, abaloparatide (brand name Tymlos) and romosozumab (brand name Evenity) have been approved by the FDA for the treatment of osteoporosis.
Abaloparatide is a novel 34-amino acid peptide, which is a selective activator of the parathyroid hormone receptor type 1 signaling pathway.1 In Phase 3 studies, women were randomized to receive abaloparatide, teriparatide, or placebo. Compared to placebo, women randomized to abaloparatide had a 3.4% increase in BMD at the total hip, 2.9% increase at the femoral neck, and 9.2% at the lumbar spine.2 These increases were observed earlier than in the teriparatide group. Abaloparatide also reduced vertebral fracture and nonvertebral fracture risk by 86% and 43%, respectively.2 Abaloparatide is effective at increasing BMD and reducing fracture risk at all levels of baseline risk (i.e., baseline BMD score, prevalent fracture status, prior fracture status, age)3 and even in the oldest old.4
Romosozumab, FDA approved April 9, 2019, is a monoclonal antibody to sclerostin. Sclerostin is produced by osteocytes and inhibits bone formation while enhancing bone resorption. The pivotal Phase 3 study showed that 1-year of romosozumab administered monthly compared to placebo increased BMD at the lumbar spine by 13%, and reduced the risk of new vertebral fractures by 73%.5 In an additional study that compared romosozumab to alendronate, the risk of new vertebral fracture was 48% lower in the group randomized to romosozumab followed by alendronate than in the group who were randomized to alendronate and continued alendronate throughout the study.6 This study, but not a larger one against placebo, found a higher rate of adverse cardiovascular safety outcomes, including cardiac ischemic events and cerebrovascular events in the romosuozumab group.6
Having one fragility fracture increases the risk for a second fragility fracture, with estimates ranging from 37% at the same site to nearly seven-fold for different sites.7,8,9,10 Thus, secondary fracture prevention is important clinically and a major emphasis of the American Society for Bone and Mineral Research (ASBMR).
Programs and Services to Help Prevent Secondary Fractures
Many tools have been created to assist in secondary fracture prevention, with the Fracture Liaison Service (FLS) programs the most well-known. FLS programs have been shown to be successful in many European countries and have become international platforms for secondary fracture prevention. In the US, the first FLS programs were initiated within the Kaiser Permanente health system, a closed health network. In 2014, a simulation analysis estimated the cost effectiveness of FLS programs if initiated in the traditional US open-healthcare system.11 This study projected that over the remaining life span (mean of 8.6 years) of 10,000 men and women who sustained an index hip fracture, there would be an estimated 5,579 subsequent fractures, including 1,958 hip, 453 distal forearm, 998 vertebral, and 2,170 fractures of other sites. Implementation of a universal FLS was projected to reduce the rates of subsequent hip fracture by 109 per 10,000 persons, 21 fewer spine fractures, 5 fewer distal forearm fractures, and 17 fewer other osteoporotic fractures. The reduction in fracture rate was associated with a $66,879 reduction in costs and an increase in quality adjusted life expectancy (QALE) of 37.4 years for every 10,000 patients.10
This information led groups, including the National Osteoporosis Foundation (NOF) and the American Orthopaedic Association’s “Own the Bone” (AOA-OTB) program, to promote FLS training in the US. The NOF’s Fracture Prevention Central (FPC) website, an online toolkit accessible to the public, includes tools and resources for those interested in starting an FLS program, as well as guidance to hospital administrators, affordable healthcare organizations, and health insurers on how to make a financial business case for implementing a FLS program at their location. NOF also has the FLS Model of Care Training Program designed to help doctors, nurse practitioners, physician assistants, registered nurses, and other healthcare professionals improve the care management of post-fracture patients and navigate the complicated coordination of the care process across hospitals, medical offices and multiple medical specialties through the application of best practices. The program is designed as a self-paced, on-demand webinar series. Participants can choose to complete individual FLS sessions or complete all sessions and receive the FLS Certificate. The goal of the program is to ensure that fracture patients receive appropriate osteoporosis testing, diagnosis, treatment and ongoing support after they leave the hospital.
Own the Bone program is a national post-fracture, systems-based, multidisciplinary fragility fracture prevention initiative. Its Web-based program and 10 prevention measures transform the way hospitals treat fracture patients. The ultimate goal is to change physician and patient behavior to reduce incidence of future fractures and positively impact osteoporosis treatment. The program conducts educational webinars and symposiums addressing nutrition counseling, physical activity counseling, lifestyle counseling, pharmacotherapy recommendations, BMD testing, and communication about bone health and additional risk factors. Own the Bone also has an extensive registry, which is consistent with the demographics of the general population of those with osteoporosis. Bone health experts establish standards of care through a medical advisory board for Own the Bone.
Both NOF and AOA-OTB have partnered with Project ECHO to disseminate FLS training through the ECHO platform, an initiative of the University of New Mexico, which uses case-based clinical discussions in a spoke and hub manner to disseminate information. The ECHO model™ breaks down the walls between specialty and primary care by linking expert specialist teams at an academic ‘hub’ with primary care clinicians in local communities – the ‘spokes’ of the model. In addition to the University of New Mexico, the ECHO platform is used by MNI Great Lakes ECHO in MI. To learn more about joining the Bone Health ECHO® program, click here.
The International Osteoporosis Foundation has the “Capture the Fracture” program, which has created the Best Practice Framework, an international benchmark for FLS programs, that includes 13 globally-endorsed standards:
1 Patient identification,
2 Patient evaluation,
3 Post fracture assessment timing,
4 Vertebral fracture,
5 Assessment guidelines,
6 Secondary causes of osteoporosis,
7 Fall prevention,
8 Multifaceted health and lifestyles risk-factor assessment,
9 Medication initiation,
10 Medication review,
11 Communication strategy,
12 Long-term management, and
13 Creating a database.
The American Society for Bone and Mineral Research (ASBMR) has created the Secondary Fracture Prevention Initiative, a coalition of bone health experts, including healthcare professionals and patient advocates, dedicated to reducing the number of avoidable second fractures in individuals with osteoporosis. The Initiative, with consensus from a broad multi-stakeholder coalition, has developed five fundamental clinical recommendations and seven additional recommendations for clinical care for women and men, age 65 years or older, with a hip or vertebral fracture. They are directed to all healthcare professionals who participate in the care of these patients, with the goal of reducing secondary fractures.
|
ICD-9-CM Codes |
ICD-10-CM Conversion Codes |
New ICD-10-CM Codes |
||
|
OSTEOPOROSIS |
||||
|
Osteoporosis unspecified: 733.00 |
M81.0 |
Age-related osteoporosis without current pathological fracture: M81.0 |
||
|
Senile osteoporosis: 733.01 |
M81.0 |
Localized osteoporosis [Lequesne]:M81.6 |
||
|
Idiopathic osteoporosis: 733.02 |
M81.8 |
Other osteoporosis without current pathological fracture: M81.8 |
||
|
Disuse osteoporosis: 733.03 |
M81.8 |
Adult osteomalacia: M83.x |
||
|
Other osteoporosis: 733.09 |
M81.8 |
|||
|
FRAGILITY FRACTURES |
||||
|
Hip fracture: 820.0, 820.2, 733.14 |
S72.019A, S72.023A, S72.033A, S72.043A, S72.099A, S72.109A, S72.143A, S72.23XA, M84.459A |
Osteoporosis with current pathological fracture: M80.x |
||
|
Spine fracture: 805.0, 805.2, 805.4, 805.8, 806.0, 806.2, 806.4, 806.8, 733.13 |
S12.9XXA, S12.000A, S12.001A, S12.100A, S12.101A, S12.200A, S12.201A, S12.300A, S12.301A, S12.400A, S12.401A, S12.500A, S12.501A, S12.600A, S12.601A, S22.009A, S32.009A, S32.10XA, S32.2XXA, S14.101A, S14.102A, S14.103A, S14.104A, S14.111A, S14.112A,S14.113A, S14.114A, S14.121A, S14.122A, S14.123A, S14.124A, S14.131A, S14.132A, S14.133A, S14.134A, S14.151A, S14.152A, S14.153A, S14.154A, S14.105A,S14.106A, S14.107A, S14.115A, S14.116A, S14.117A, S14.125A, S14.126A, S14.127A, S14.135A, S14.136A, S14.137A, S14.155A, S14.156A, S14.157A, S24.101AS24.102A, S24.111A, S24.112A, S24.131A, S24.132A, S24.151A, S24.152A, S24.103A, S24.104A, S24.113A, S24.114A, S24.133A, S24.134A, S24.153A, S24.154A,S34.109A, S34.119A, S34.129A, S32.009A, S34.101A, S34.111A, S34.121A, S32.019A, S34.102A, S34.112A, S34.122A, S32.029A, S34.103A, S34.113A, S34.123A,S32.039A, S34.104A, S34.114A, S34.124A, S32.049A, S34.105A, S34.115A, S34.125A, S32.059A, S14.109A, S24.109A, S34.109A, S34.139A, M48.50XA, M80.08XA,M84.48XA, M84.68XA |
Stress fracture: M84.3 |
||
|
Pelvic fracture: 808.0, 808.2, 808.4, 808.8 |
S32.409A, S32.501A, S32.501A, S32.509A, S32.309A, S32.609A, S32.810A, S32.811A, S32.82XA, S32.89XA, S32.9XXA |
Pathological fracture, not elsewhere classified: M84.4 |
||
|
Femur (thigh) fracture: 821.0, 821.2, 733.15 |
S72.90XA, S72.309A, S72.409A, S72.413A, S72.416A, S72.443A, S72.446A, S72.453A, S72.456A, S72.499A, M84.453A |
|||
|
Wrist fracture: 813.4, 733.12 |
S52.90XA, S52.539A, S52.549A, S52.509A, S52.609A, S52.119A, S52.529A, S52.019A, S52.629A, S52.011A, S52.012A, S52.621A, A52.622A, M84.439A |
|||
|
Humerus (arm) fracture: 812.0, 812.2, 812.4, 733.1 |
S42.209A, S42.213A, S42.216A, S42/293A, S42.295A, S42.253A, S42.256A, S42/293A, S42.296A, S42.309A, S42.399A, S42.409A, S42.413A, S42.416A, S42.433A, S42.436A, S42.453A, S42.456A, S42.443A, S42.446A, S42.463A, S42.466A, S42.473A, S42.476A, S42.493A, S42.496A, M84.40XA |
|||