 [7]
[7] 

Inflammatory arthritis is a group of diseases characterized by inflammation of the synovial membrane in the joints and, often, other tissues throughout the body. Some forms of inflammatory arthritis are autoimmune diseases, conditions in which the body’s immune system attacks healthy tissue, also known as systemic autoimmune rheumatic diseases (SARD). Examples of SARDs that cause inflammatory arthritis include rheumatoid arthritis (RA), systemic lupus erythematosus (SLE), Sjögren’s syndrome (SjS), systemic sclerosis (SSc), polymyositis (PM), and dermatomyositis (DM). Other types of inflammatory arthritis include axial spondyloarthritis (formerly called ankylosing spondylitis) and psoriatic arthritis, along with gout which is also considered a metabolic arthritis and discussed under it's own heading.
As a group, inflammatory arthritic diseases are characterized by joint pain, swelling, warmth, and tenderness in joints, and can cause deformity and loss of function of affected joints. Since these diseases are systemic, they may be associated with involvement of other tissues or organs including the skin, eye and bowel. In addition, in these diseases, blood tests provide evidence of inflammation and some conditions are useful markers that assess disease likelihood. Inflammatory arthritis conditions are sometimes difficult to diagnose and distinguish; all patients suspected of having an inflammatory arthritis should be referred to a rheumatologist for evaluation and management. Arthritis occurring in children and adolescents is referred to as juvenile idiopathic arthritis (formerly juvenile rheumatoid arthritis) and is discussed in the Juvenile Arthritis [1] heading.
Only the most common inflammatory arthritides will be discussed below. A listing of the many types of inflammatory arthritis and related conditions can be seen by clicking HERE [2].
Rheumatoid arthritis (RA) is a systemic autoimmune disease that produces inflammatory arthritis (stiff, painful, swollen joints, usually symmetrical). Rheumatoid arthritis is a form of polyarthritis and involves many joints, both large and small; it can also affect the cervical spine. Over time, RA can affect other organs (eg, eyes, lungs) and can lead to increased risk of cardiovascular disease.1
Rheumatoid arthritis is a chronic condition and, while it may occur acutely in some patients, onset is usually gradual. It can take months before a patient seeks medical attention, usually when joint pain (arthralgia) progresses to swelling and tenderness of the joint. As a systemic disease, RA is associated with symptoms such as fatigue, weight loss, and depression. In RA, inflammation of the joint can lead to erosion or damage of cartilage and bone and eventual deformity. The patient with RA characteristically produces autoantibodies called rheumatoid factors and anti-CCP. Anti-CCP (cyclic citrullinated peptide) antibodies are directed to proteins that have a modified amino acid called citrulline. These antibodies occur in approximately 70-80% of patients and are important for diagnosis and early recognition.
Rheumatoid arthritis was historically categorized based on the American Rheumatism Association Functional Class and Anatomic Stage, both proposed by Dr. Otto Steinbrocker in 1949. The former was updated by the American College of Rheumatology (ACR) in 19922 as follows:
Class I: Patient able to perform usual activities of daily living (self-care [dressing, feeding, bathing, grooming, and toileting], vocational [work, school, or homemaking] and avocational [recreational and/or leisure])
Class II: Able to perform usual self-care and vocational activities, but limited in avocational activities
Class III: Able to perform usual self-care activities but limited in vocational and avocational activities
Class IV: Limited in ability to perform usual self-care, vocational and avocational activities.
The revised classes were validated in a study of 325 patients using the Health Assessment Questionnaire (HAQ): mean HAQ disability index scores were Class I = 0.33, Class II = 1.02, Class III = 1.70 and Class IV = 2.67.
It is currently the usual practice to consider staging RA based on duration of signs and symptoms and the presence of autoantibodies and radiographic erosions. Hence, as currently defined by the ACR, RA is classified as follows:
Early RA = Signs and symptoms of < 6 months duration
Established RA = Signs and symptoms of ≥ 6 months duration or meeting the 1987 classification criteria
Seropositivity = presence of either rheumatoid factor (RF) or anti-citrullinated peptide antibodies (ACPA). Presence of erosions on radiographs of the hands/wrists.
In addition, one considers the level of disease activity at the time of the patient’s visit to inform treatment decisions. Several reliable and valid instruments are available for this purpose; most useful are the Disease Activity Score 28 [3] using either the erythrocyte sedimentation rate or the C-reactive protein marker, the Simplified Disease Activity Index [4], or the Clinical Disease Activity Index [5]. The latter does not require obtaining any laboratory tests to measure acute phase reactants. The ACR has published recommendations for the management of RA based on the above parameters, especially disease duration and disease activity.3
Although there is no cure for RA, early identification and treatment is important since current therapy can lead to significant improvement and reduce the likelihood for joint damage and progression to deformity. Therapy for RA involves a large group of medications that decrease inflammation and modify the course of disease. These agents are called DMARDS (disease modifying antirheumatic drugs) and have led to important improvement in overall outlook.
Prevalence of Rheumatoid Arthritis
As noted earlier in this report, clinical data are required to provide validity for estimating the prevalence of specific types of arthritis because the exact type of AORC causing pain and swelling is often unclear from observation. Prevalence of RA in the US is estimated to be between 1.3 and 1.5 million persons,4,5,6 roughly 0.50% of the adult population. Prevalence varies by sex, affecting 0.29%-0.31% of males and 0.73%-0.78% of females.6Also note that prevalence varies by age with highest ratios in older adults aged 65 years and older and lower ratios in declining 10-year age groups. The estimated prevalence of RA in the US population age 60 years and older is 2%.7
Healthcare Utilization
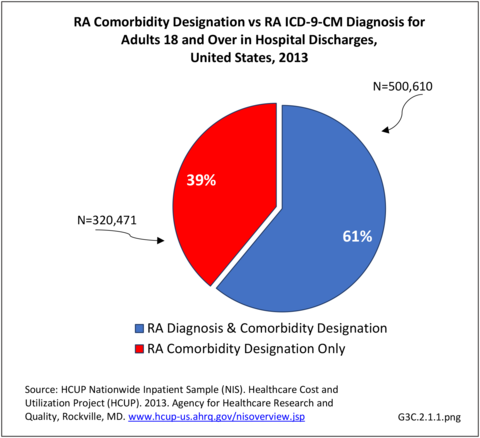
Rheumatoid arthritis effects overall health but may not be identified as the condition for which a patient is hospitalized. The NIS includes a separate variable identifying comorbidities of patients. Analyzing this variable, RA was identified as a comorbidity in 821,100 hospital discharges, or 2.7% of all hospital discharges, in 2013. However, when discharges were analyzed using the ICD9-CM codes, RA was diagnosed in only 512,600 discharges, or 1.7% of discharges for any diagnoses. Comorbidity designations are not made for all inpatients. Overall, 61% of discharges with RA diagnosed as a comorbidity also had an admitting diagnosis of RA, leaving two in five (39%) diagnosed with RA as a comorbidity but hospitalized for another cause. Common other forms of arthritis and associated diseases with RA as a comorbid condition include lupus (SLE) and fibromyalgia. (Reference graphs G3C.2.1.1 and G3C.2.1.2)
As previously noted, RA was diagnosed in slightly more than one-half million hospitalizations in 2013, representing 1.7% of discharges for any diagnoses. This is compared with the general prevalence rate of approximately 0.5%. Mean length of hospital stay and mean hospital charges were slightly higher than for all hospital discharges (106% and 109%, respectively). Nearly half (45%) of discharges with an RA diagnoses were dischared to additional care (short-term or home health), compared with 33% for all diagnoses discharges. (Reference Table 3A.3.1.0.1 PDF [10] CSV [11]; Table 3A.3.1.1.1 PDF [12] CSV [13]; Table 3A.3.1.3.1 PDF [14] CSV [15])
Rheumatoid arthritis was the first diagnoses recorded in 1.4% of total hip replacements and 0.3% of total knee replacements in 2013. (Reference Table 3A.5.3 PDF [18] CSV [19])
For RA, females outnumbered males three to one. Most RA hospitalizations occurred in those aged 65 years and older at a rate of 0.7 adults in 100 for this age group. No differences in rates were found by race/ethnic or regional group. (Table 3A.3.1.0.1 PDF [10] CSV [11]; Table 3A.3.1.0.2 PDF [20] CSV [21]; Table 3A.3.1.0.3 PDF [22] CSV [23]; Table 3A.3.1.0.4 PDF [24] CSV [25])
Rheumatoid arthritis was diagnosed in 6.4 million ambulatory visits and accounted for 0.7% of ambulatory care visits with an arthritis diagnosis, compared with the 0.5% prevalence rate in the US population. An RA diagnosis was made in 0.6% of physician office visits and ER visits; 0.8% of outpatient visits had a RA diagnosis. (Reference Table 3A.3.2.0.1 PDF [26] CSV [27]; Reference Table 3A.3.2.1.1 PDF [28] CSV [29]; Table 3A.3.2.2.1 PDF [30] CSV [31]; and Table 3A.3.2.3.1 PDF [32] CSV [33])
The distribution of ambulatory care visits by select demographic characteristics, when compared to all ambulatory visits for RA, was highest among females, and lowest among those younger than age 44 and among Black non-Hispanic and Hispanic racial/ethnic groups. (Reference Table 3A.3.2.0.1 PDF [26] CSV [27]; Table 3A.3.2.0.2 PDF [34] CSV [35]; Table 3A.3.2.0.3 PDF [36] CSV [37]; Table 3A.3.2.0.4 PDF [38] CSV [39])
Economic Burden
Estimates were calculated from 2008-2012 Medical Expenditures Panel Survey (MEPS) data; analysis was limited to those years because the ICD-9-CM code for RA was suppressed in the 2013 and 2014 MEPS data. MEPS respondents were classified as having RA if they met the following criteria: had a record with ICD-9-CM code 714, self-reported having ever been diagnosed with RA, and had at least five prescriptions or ambulatory care visits for RA. In the 2008–2012 period, each year, an estimated 1.7 million adults (0.8% of US adult population) had RA. Although slightly higher than the 1.3 million to 1.5 million previously cited by other sources, the numbers provide a similar rate of RA in the adult population.
Combining direct and indirect costs for RA, total average costs annually for the years 2008-2014 were $46 billion, with incremental costs, those costs directly associated with RA, of $21.6 billion. (Reference Table 8.13 PDF [42] CSV [43])
Annual average per person all-cause (diagnosis of RA along with other health condition diagnoses) medical expenditures for RA were $19,040. Across selected characteristics, the five groups with the highest all-cause per person costs were those who were college graduates ($25,526); had any limitation in work, housework, or school activities ($25,220); lived in the Northeast ($24,038); Hispanics ($22,871), and those with any limitation in IADLS, ADLs, functioning, work, housework, school, vision or hearing ($21,858). Lowest per person costs were among the uninsured ($8,674) and across the remaining subgroups, average per person costs were at least $14,387.
Total all-cause medical expenditures were $32.9 billion. Total costs include ambulatory care, inpatient care, prescriptions filled, and residual costs (ER, home health, medical devices).
For incremental medical expenditures (expenditures directly attributed to RA), mean per person expenditures for RA averaged $7,957 for the years 2008-2012. Aggregate medical expenditures (combined cost for all persons) in the United States for RA averaged $13.8 billion in each of the years of 2008-2012. (Reference Table 8.13 PDF [42] CSV [43] and Table 8.23 PDF [48] CSV [49])
The ratio of persons in the labor force without RA is higher than for those with RA in the general population, resulting in earnings losses due to RA. Among the estimated 900,746 working age adults (18-64 years) with a work history and RA, 56.1% had worked during the year compared with 87.9% of those without RA. Each year, those with RA earned, on average, $14,542 less than those without RA, which among all adults with RA totaled $13.1 billion.
For incremental medical expenditures, mean per person earnings losses attributed to RA averaged $8,748 per year in 2008-2012. Aggregate earnings losses for the United States due to RA averaged $7.9 billion in each of the years of 2008-2012. (Reference Table 8.13 PDF [42] CSV [43] and Table 8.23 PDF [48] CSV [49])
The cost of treating RA can be high. Older treatments of NSAIDS (aspirin, ibuprofen, naproxen, and celecoxib) and analgesics (acetaminophen, morphine, oxycodone) are readily available and inexpensive. However, many who suffer from RA cannot tolerate these drugs or they do not suppress the pain. A second level of drugs, the DMARDs (disease-modifying antirheumatic drugs) designed to reduce symptoms and damage, have become more affordable than previously, but still cost between $1,500 and $2,000 annually.
The newest level of drugs, the biologics, remain very expensive. Biologics are genetically engineered proteins originating from human genes targeting specific parts of the immune system that fuel inflammation. The first biologic, etanercept (Enbrel), was approved in 1998, and was used to treat RA. Actual cost estimates have a wide range, an average $18,000 to $100,000 annually, depending on the type of biologic used.8,9,10,11 In addition, because most are administered through an IV or injection administered by a healthcare professional, there are additional costs. Higher medication costs have been found to be associated with age and comorbidities.12
Spondyloarthropathy (SpA) refers to a family of inflammatory arthropathies that primarily affect the vertebral column. This group differs from other types of arthritis, especially rheumatoid arthritis, in that, rather than primarily affecting the synovial lining tissue in the joints, it involves the connective tissue where the tendons and ligaments attach to bone (entheses). Furthermore, patients with these disorders usually have negative tests for both rheumatoid factor and antibodies to citrullinated peptides (autoantibodies seen in the majority of patients with RA), often have radiographic involvement of the sacroiliac joints, and may have ocular inflammation (i.e., acute iritis or uveitis). Symptoms are often termed inflammatory back pain which is gradual in onset, worse in the morning and improves with activity. Inflammation can also affect the large joints of the lower extremities, including the knees and ankles. In the spondyloarthropathies, sacroiliac joints can fuse, and new bone can form between vertebrae. This leads to ankylosing and can cause deformity of the spine. In some patients, the spine can become rigid.
Among the conditions included in the SpA family, axial spondyloarthritis (formerly known as ankylosing spondylitis [AS]) is the most common and refers to inflammation of the spine or one or more adjacent structures of the vertebrae. Axial spondyloarthritis causes inflammation of the tissues in the spine and the root joints (shoulders and hips) and may be associated with peripheral arthritis. Over time, patients can undergo fusion of the vertebrae, limiting movement. Axial spondyloarthritis has a hereditary component and runs in families. It affects males more than females and can occur at any age. Patients with SpA frequently have a genetic marker called HLA B27. Since HLA B27 occurs commonly in the otherwise healthy population (approximately 8% of the US), it is not used as a specific diagnostic marker. HLA B27 is less common in African Americans.
In addition to AS, the more common diseases in the (SpA) family are:
• Reactive arthritis (formerly known as Reiter’s syndrome), a reaction to an infection in another part of the body;
• Psoriatic arthritis, which can occur in people with the skin disease psoriasis; and
• Enteropathic arthritis/spondylitis, a form of chronic inflammatory arthritis associated with inflammatory bowel diseases such as ulcerative colitis and Crohn’s disease. Enteropathic arthritis may be designated as axial (low back pain due to ankylosing spondylitis) or peripheral (joint involvement).
While some patients with psoriatic arthritis have a spondyloarthritis, in others, the involvement is primarily in peripheral joints. Psoriatic arthritis can resemble RA, but tests for rheumatoid factor and anti-CCP will be negative.
Prevalence of Spondylarthropathies
The prevalence of SpA in the US is difficult to determine as the diseases affect ethnic groups differently. Estimates of prevalence for SpA are 0.01%-2.5%.1,2 Current estimates of prevalence of the more common diseases are:
• Ankylosing spondylitis, 0.2%-1.7%1,2,3,4
o Axial SpA, 0.9%-1.4%2,3,4,5,6
o Advanced AS, 0.52%-0.55%2
• Psoriatic arthritis, 0.1%-0.4%1
• Reactive arthritis, no estimate found
• Enteropathic peripheral arthritis, 0.065%1
• Enteropathic axial arthritis, 0.05%-0.25%.1
Healthcare Utilization
Spondyloarthropathy was diagnosed in about one-half million hospitalizations in 2013, representing 1.6% of hospital discharges for all diagnoses, a higher proportion than prevalence in the population (1.6% of discharges vs 1.0%). No differences were found by sex, race/ethnicity, or geographic region, but age was a factor in the rate of hospitalizations for SpA. (Table 3A.3.1.0.1 PDF [10] CSV [11]; Table 3A.3.1.0.2 PDF [20] CSV [21]; Table 3A.3.1.0.3 PDF [22] CSV [23]; Table 3A.3.1.0.4 PDF [24] CSV [25])
Among those with a diagnosis of SpA, hospital discharge rates showed higher mean charges ($60,000 per SpA discharge versus $43,000 for any diagnoses) for a similar mean length of stay (4.6 days versus 4.7 days). Discharges from the hospital to additional care (short-term or home health) was slightly higher for persons with a diagnois of SpA (40%) than for all diagnoses discharges (31%). (Reference Table 3A.3.1.1.1 PDF [12] CSV [13]; Table 3A.3.1.3.1 PDF [14] CSV [15])
Spondylarthropathies accounted for 0.7% of all diagnoses ambulatory care visits. Males were slightly more likely (0.8%) to receive ambulatory health care for SpA than females, along with those age 45 to 64 years (1.0%) and those living in the South (0.9%). (Reference Table 3A.3.2.0.1 PDF [26] CSV [27]; Table 3A.3.2.0.2 PDF [34] CSV [35]; Table 3A.3.2.0.3 PDF [36] CSV [37]; Table 3A.3.2.0.4 PDF [38] CSV [39])
Economic Burden
Economic burden was not calculated by the BMUS project for spondyloarthropathies due to sample sizes. One study cited mean annual direct medical costs for AS of $6,500.7
Several published studies have explored the medication cost of biologics. For AS, biologic cost ranged from $1,200 to $24,200; for PSA ranged $14,200 to $32,000.7,8,9
Connective tissue disorders (CTDs) are part of the systemic autoimmune rheumatic diseases (SARD) grouping of disorders and include systemic lupus erythematosus (SLE or lupus), systemic sclerosis (SSc or scleroderma), inflammatory myositis (polymyositis and dermatomyositis), and Sjögren syndrome (SjS). They are characterized by a heterogeneous group of immune-mediated inflammatory signs and symptoms affecting multiple organ systems, including the joints.
Prevalence of Connective Tissue Disorders
The prevalence of syndromes in the CTD family are difficult to identify, and vary depending on the study duration, classification criteria, and the country in which the study was undertaken. Current estimates are based on special populations and primarily use several CDC-funded state registries. Connective tissue disorders affect all ages, but incidence is higher among women than men by a factor of at least 4:1, with estimates as high as 12:1 for SLE.1,2 Lupus generally begins during women’s children bearing years and can lead to serious kidney involvement among other complications.
The highest prevalence is for SjS, ranging between 0.5% and 3% of a given demographic population.1 Estimates of overall prevalence range from 400,000 to 3.1 million US adults.3
Recent national estimates of prevalence and incidence of SLE in the US are not available, but it is relatively uncommon. Using older meta-analysis studies, prevalence of SLE is estimated between 15 and 50 per 100,000 individuals.1 The Lupus Foundation of America estimates a total of 1.5 million Americans have some form of lupus, with an incidence of 16,000 new cases per year.4
The prevalence of SSc, also known as scleroderma, is much lower and has been reported with an incidence of 20 per one million new cases per year and a prevalence of 240 per million US adults, based on a limited US population studies published in 2003.5,6 A more recent update did not find this estimate to be changed.7
Overall prevalence and incidence of CTD is not reported in the literature, as classification criteria are not defined.1 However, the economic analysis for this report places prevalence at 0.27% for the years 2008 thru 2014. (Reference Table 8.20 PDF [62] CSV [63])
Healthcare Utilization
Connective tissue disorders represented 1% of hospital discharges and total charges for all diagnoses hospital stays in 2013. Because of the very low incidence of CTD syndromes, the prevalence is estimated at 0.3 percent or less, with use of healthcare resources much higher than the incidence ratio. Although the share of hospital discharges is higher than the share of all ambulatory visits, the rate of hospital discharges per 100 adults is much lower than the rate of ambulatory visits. Mean length of hospital stay and mean hospital charges are slightly higher than the means for all diagnoses, but patients are generally discharged to home self-care. (Reference Table 3A.3.1.0.1 PDF [10] CSV [11]; Table 3A.3.2.0.1 PDF [26] CSV [27]; Table 3A.3.1.1.1 PDF [12] CSV [13]; Table 3A.3.1.3.1 PDF [14] CSV [15])
Hospitalizations for CTDs occurred primarily in females, those age 45-64 years, and non-Hispanic blacks compared to all diagnoses discharges. (Table 3A.3.1.0.1 PDF [10] CSV [11]; Table 3A.3.1.0.2 PDF [20] CSV [21]; Table 3A.3.1.0.3 PDF [22] CSV [23]; Table 3A.3.1.0.4 PDF [66] CSV [25])
Connective tissue disorders accounted for 0.4% of all diagnoses for ambulatory care visits. The distribution of ambulatory care visits by select demographic characteristics, when compared to all ambulatory visits for CTDs, was highest among females and non-Hispanic blacks, and lowest among those aged 65 years and older and those living in the Midwest. Females accounted for nearly all ambulatory CTD visits. (Reference Table 3A.3.2.0.1 PDF [26] CSV [27]; Table 3A.3.2.0.2 PDF [34] CSV [35]; Table 3A.3.2.0.3 PDF [36] CSV [37]; Table 3A.3.2.0.4 PDF [69] CSV [39])
Data from the MEPS, used exclusively in the economic analysis of this report, show higher levels of healthcare visits than the NIS and NAMCS. In particular, the number of ambulatory physician visits is much higher in the MEPS than in the NAMCS. Differences in how conditions are classified and data coded account for some of this, as does the inclusion of ambulatory visits in settings outside a physician’s office (eg, ER or outpatient clinic).
Based on the MEPS, most individuals with a CTD (93%) incurred one or more ambulatory physician visits; among all of those with CTD, the total number of ambulatory physician visits was 9.3 million visits (average visits per person=11.2) annually. Approximately two-thirds (67.9%) of those with CTDs had at least one non-physician care visit, which include physical therapists and alternative care. The average number of non-physician visits per person was 8.5, for a total of 7.0 million non-physician visits nationally. One in five (20.7%) individuals with a CTD were hospitalized and there were 300,000 hospitalizations among all people with CTDs, with an average of 0.4 hospitalizations per person. The percentage with home health care visits was lower (14.4%) than for the other types of visits. However, those with a CTD had an average of 17.1 home health care visits per year for a total of 14.2 million visits nationally. Furthermore, those who did have a home health visit had very high home health visit utilization, with an average of 119 visits annually (data not shown). Finally, almost all individuals with a a CTD filled a prescription medication (95.8%); the total number of prescription fills each year was 39.5 million, based on average prescription fills among all of those with CTD of 47.7 fills. (Reference Table 8.20 PDF [62] CSV [63])
Economic Burden
From 2008-2014, an estimated 800,000 individuals (0.27%) in the US population had a CTD annually. Across all age groups, middle age adults (45-64 years) represented the largest percentage of those with a CTD (52% or 430,000), followed by younger adults (18-44 years) (26% or 219,000 individuals), older adults (≥ 65 years) (21% or 173,000) and children (18 years) (1% or 8,000 individuals). These numbers translate into prevalence rates shown in the graph below. (Reference Table 8.19 PDF [72] CSV [73]; Table 8.21 PDF [74] CSV [75])
Females comprised the majority of those with a CTD (767,000); at least 400,000 individuals in the following groups had a CTD: those with any limitation in IADLS, ADLs, functioning, work, housework, school, vision, or hearing (597,000); non-Hispanic Whites (542,000); those with any private insurance (503,000); and those with any limitation in work, housework, or school activities (453,000). (Reference Table 8.21 PDF [74] CSV [75])
Among all individuals with a CTD, ambulatory care represented 32% of all direct costs, followed by inpatient care (28%), prescriptions (25%), and residual costs (15%). The distribution across service category varied substantially across socio-demographic and health status characteristics suggesting very different treatment and utilization patterns across these groups. For example, there were regional differences: among those in the Northeast, ambulatory care, inpatient care, prescriptions, and other costs represented 40%, 11%, 21%, and 28%, respectively whereas in the Midwest, these categories represented 25%, 24%, 44%, and 7% of all costs, respectively.
Among all individuals with a CTD, all-cause annual per person costs were $19,702. The five groups with the highest all-cause per person costs were those who were college graduates ($30,471), had public health insurance only ($29,579), lived in the Northeast ($27,349), had never married ($27,026), or reported any limitation in work, housework, or school activities ($27,024).
The five groups with the lowest all-cause per person costs were those with no health insurance ($5,631), lived in the Midwest ($11,821), were Non-Hispanic black ($14,564), had a high school education but no college education ($14,617), or were married/had a partner ($14,735). (Reference Table 8.21 PDF [74] CSV [75])
Indirect costs (earnings losses) were not calculated for CTDs due to small numbers of cases.
Links:
[1] http://www.boneandjointburden.org/fourth-edition/iiib60/juvenile-arthritis
[2] https://bmus.latticegroup.com/docs/bmus_e4_Conditions%20Related%20to%20Inflammatory%20Arthritis.pdf
[3] https://www.nras.org.uk/the-das28-score
[4] https://www.rheumatology.org/Portals/0/Files/SDAI%20Form.pdf
[5] https://www.rheumatology.org/Portals/0/Files/CDAI%20Form.pdf
[6] https://bmus.latticegroup.com/file/bmuse4g3c211png
[7] https://bmus.latticegroup.com/docs/bmus_e4_g3c.2.1.1_0.png
[8] https://bmus.latticegroup.com/file/bmuse4g3c212png
[9] https://bmus.latticegroup.com/docs/bmus_e4_g3c.2.1.2_0.png
[10] https://bmus.latticegroup.com/docs/bmus_e4_t3a.3.1.0.1.pdf
[11] https://bmus.latticegroup.com/docs/bmus_e4_t3a.3.1.0.1.csv
[12] https://bmus.latticegroup.com/docs/bmus_e4_t3a.3.1.1.1.pdf
[13] https://bmus.latticegroup.com/docs/bmus_e4_t3a.3.1.1.1.csv
[14] https://bmus.latticegroup.com/docs/bmus_e4_t3a.3.1.3.1.pdf
[15] https://bmus.latticegroup.com/docs/bmus_e4_t3a.3.1.3.1.csv
[16] https://bmus.latticegroup.com/file/bmuse4g3c213png
[17] https://bmus.latticegroup.com/docs/bmus_e4_g3c.2.1.3_0.png
[18] https://bmus.latticegroup.com/docs/bmus_e4_t3a.5.3.pdf
[19] https://bmus.latticegroup.com/docs/bmus_e4_t3a.5.3.csv
[20] https://bmus.latticegroup.com/docs/bmus_e4_t3a.3.1.0.2.pdf
[21] https://bmus.latticegroup.com/docs/bmus_e4_t3a.3.1.0.2.csv
[22] https://bmus.latticegroup.com/docs/bmus_e4_t3a.3.1.0.3.pdf
[23] https://bmus.latticegroup.com/docs/bmus_e4_t3a.3.1.0.3.csv
[24] https://bmus.latticegroup.com/docs/bmus_e4_t3a.3.1.0.4.pdf
[25] https://bmus.latticegroup.com/docs/bmus_e4_t3a.3.1.0.4.csv
[26] https://bmus.latticegroup.com/docs/bmus_e4_t3a.3.2.0.1.pdf
[27] https://bmus.latticegroup.com/docs/bmus_e4_t3a.3.2.0.1.csv
[28] https://bmus.latticegroup.com/docs/bmus_e4_t3a.3.2.1.1.pdf
[29] https://bmus.latticegroup.com/docs/bmus_e4_t3a.3.2.1.1.csv
[30] https://bmus.latticegroup.com/docs/bmus_e4_t3a.3.2.2.1.pdf
[31] https://bmus.latticegroup.com/docs/bmus_e4_t3a.3.2.2.1.csv
[32] https://bmus.latticegroup.com/docs/bmus_e4_t3a.3.2.3.1.pdf
[33] https://bmus.latticegroup.com/docs/bmus_e4_t3a.3.2.3.1.csv
[34] https://bmus.latticegroup.com/docs/bmus_e4_t3a.3.2.0.2.pdf
[35] https://bmus.latticegroup.com/docs/bmus_e4_t3a.3.2.0.2.csv
[36] https://bmus.latticegroup.com/docs/bmus_e4_t3a.3.2.0.3.pdf
[37] https://bmus.latticegroup.com/docs/bmus_e4_t3a.3.2.0.3.csv
[38] https://bmus.latticegroup.com/docs/bmus_e4_t3a.3.2.0.4.pdf
[39] https://bmus.latticegroup.com/docs/bmus_e4_t3a.3.2.0.4.csv
[40] https://bmus.latticegroup.com/file/bmuse4g3c214png
[41] https://bmus.latticegroup.com/docs/bmus_e4_g3c.2.1.4_1.png
[42] https://bmus.latticegroup.com/docs/bmus_e4_T8.13.pdf
[43] https://bmus.latticegroup.com/docs/bmus_e4_T8.13.csv
[44] https://bmus.latticegroup.com/file/bmuse4g3c215png
[45] https://bmus.latticegroup.com/docs/bmus_e4_g3c.2.1.5_0.png
[46] https://bmus.latticegroup.com/file/bmuse4g3c216png
[47] https://bmus.latticegroup.com/docs/bmus_e4_g3c.2.1.6_0.png
[48] https://bmus.latticegroup.com/docs/bmus_e4_T8.23.pdf
[49] https://bmus.latticegroup.com/docs/bmus_e4_T8.23.csv
[50] https://bmus.latticegroup.com/file/bmuse4g3c217png
[51] https://bmus.latticegroup.com/docs/bmus_e4_g3c.2.1.7_0.png
[52] https://doi.org/10.1136/bmj.k1036
[53] https://www.bmj.com/content/361/bmj.k1036
[54] https://bmus.latticegroup.com/file/bmuse4g3c221png
[55] https://bmus.latticegroup.com/docs/bmus_e4_g3c.2.2.1_0.png
[56] https://bmus.latticegroup.com/file/bmuse4g3c222png
[57] https://bmus.latticegroup.com/docs/bmus_e4_g3c.2.2.2_0.png
[58] https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4470267/
[59] https://www.ncbi.nlm.nih.gov/pubmed/23436774
[60] http://dx.doi.org/10.7812/TPP/15-151
[61] https://www.rheumatology.org/I-Am-A/Patient-Caregiver/Diseases-Conditions/Spondyloarthritis
[62] https://bmus.latticegroup.com/docs/bmus_e4_T8.20.pdf
[63] https://bmus.latticegroup.com/docs/bmus_e4_T8.20.csv
[64] https://bmus.latticegroup.com/file/bmuse4g3c231png
[65] https://bmus.latticegroup.com/docs/bmus_e4_g3c.2.3.1_0.png
[66] https://bmus.latticegroup.com/docs/bmus_e4_t3a.3.1.0.4pdf
[67] https://bmus.latticegroup.com/file/bmuse4g3c232png
[68] https://bmus.latticegroup.com/docs/bmus_e4_g3c.2.3.2_0.png
[69] https://bmus.latticegroup.com/docs/bmus_e4_t3a.3.2.0.4pdf
[70] https://bmus.latticegroup.com/file/bmuse4g3c233png
[71] https://bmus.latticegroup.com/docs/bmus_e4_g3c.2.3.3_0.png
[72] https://bmus.latticegroup.com/docs/bmus_e4_T8.19.pdf
[73] https://bmus.latticegroup.com/docs/bmus_e4_T8.19.csv
[74] https://bmus.latticegroup.com/docs/bmus_e4_T8.21.pdf
[75] https://bmus.latticegroup.com/docs/bmus_e4_T8.21.csv
[76] https://bmus.latticegroup.com/file/bmuse4g3c234png
[77] https://bmus.latticegroup.com/docs/bmus_e4_g3c.2.3.4_0.png
[78] https://bmus.latticegroup.com/file/bmuse4g3c235png
[79] https://bmus.latticegroup.com/docs/bmus_e4_g3c.2.3.5_0.png
[80] https://bmus.latticegroup.com/file/bmuse4g3c236png
[81] https://bmus.latticegroup.com/docs/bmus_e4_g3c.2.3.6_0.png
[82] https://doi.org/10.1093/rheumatology/kel282
[83] https://www.rheumatology.org/I-Am-A/Patient-Caregiver/Diseases
[84] https://resources.lupus.org/entry/facts-and-statistics